01 April 2021: Articles 
Thromboelastography-Guided Anticoagulant Therapy for the Double Hazard of Thrombohemorrhagic Events in COVID-19: A Report of 3 Cases
Unusual clinical course, Unusual or unexpected effect of treatment
Connor M. Bunch1ABDEF, Anthony V. ThomasDOI: 10.12659/AJCR.931080
Am J Case Rep 2021; 22:e931080
Abstract
BACKGROUND: The novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), often manifests a coagulopathy in severely ill patients, which may cause hemorrhage and/or thrombosis of varying severity. This report comprises the cases of 3 patients with COVID-19-associated coagulopathy who were evaluated with thromboelastography (TEG) and activated partial thromboplastin time (aPTT) to enable personalized anticoagulant therapy.
CASE REPORT: Three patients presented with COVID-19 pneumonia, confirmed by reverse transcription-polymerase chain reaction, who developed thrombohemorrhagic coagulopathy. Case 1: A 72-year-old woman on long-term warfarin therapy for a history of venous thromboembolism developed a right upper lobe pulmonary embolus, despite an international normalized ratio of 6.4 and aPTT of 120.7 s. TEG enabled successful anticoagulation with heparin, and her pulmonary infarct was no longer present 2 weeks later. Case 2: A 55-year-old woman developed a rectus sheath hematoma while on heparin, and TEG demonstrated increased fibrinolysis despite COVID-19 patients more commonly undergoing fibrinolytic shutdown. Case 3: A 43-year-old woman had significant thrombus burden while severely hypocoagulable according to laboratory testing. As the venous thrombi enlarged in a disseminated intravascular coagulopathic-like state, the heparin dose was escalated to achieve a target aPTT of 70 to 80 s, resulting in a flat line TEG tracing.
CONCLUSIONS: These 3 cases of COVID-19 pneumonia with complex and varied clinical histories demonstrated the clinical value of TEG combined with the measurement of aPTT to facilitate personalized anticoagulation, resulting in good clinical outcomes.
Keywords: Anticoagulants, COVID-19, Heparin, Thrombelastography, venous thromboembolism, Hemorrhage, COVID-19, Thrombolytic Therapy, Thrombosis
Background
Anticoagulant dosing in hospitalized patients infected with the severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), the pathogen that causes the novel coronavirus disease 2019 (COVID-19), remains an area of controversy [1,2]. The United States’ National Institute of Health expert consensus on COVID-19 therapy currently cites insufficient data for or against the escalation of prophylactic anticoagulation to intermediate or therapeutic dosing for hospitalized patients without radio-graphically proven macrothrombosis [1]. The initial enthusiasm for intermediate or therapeutic doses was based on the high rates of venous thromboembolisms (VTEs), pulmonary embolisms (PEs), and arterial thromboses in patients with COVID-19, particularly those in the intensive care unit (ICU) [3,4]. Despite the known hypercoagulable states of these patients, bleeding may occur with standard doses of low molecular weight heparin or unfractionated heparin (UFH) [5–8]. A major US trial for therapeutic anticoagulation in critically ill patients with COVID-19 was recently halted owing to the high incidence of bleeding in patients in the ICU [9]. Providing the standard level of anticoagulation was an attractive therapeutic hypothesis, which was later shown to be potentially life-threatening [8,9].
The thrombohemorrhagic complications of COVID-19 observed early in the pandemic prompted us to form a coagulation committee tasked with adjudicating anticoagulation to simultaneously reduce the incidence of thrombosis and clinically significant hemorrhage. The viscoelastic hemostatic assays, thromboelastography (TEG) and rotational thromboelastometry (ROTEM), are attractive tests for hemostatic monitoring because of their demonstrable use across several disciplines, including trauma resuscitation, extracorporeal membrane oxygenation (ECMO), major surgery, obstetrics, and inherited and acquired coagulopathies [10–13]. Viscoelastic hemostatic assays have several advantages over the plasma-based common coagulation tests (eg, activated partial thromboplastin time (aPTT), prothrombin time). TEG/ROTEM enable the rapid identification of the specific disruption in the clotting process, as well as fibrinolysis, and have proven to be cost-effective for improving patient outcomes [13,14]. TEG/ROTEM depict a graphical representation of the entire coagulation process using whole blood [12]. TEG parameters include the reaction time (R), which represents the time to initial fibrin formation (standard range, 5–10 min). Clot kinetics are represented by clot formation (K), which indicates the speed of fibrin build up (standard range, 1–3 min), and the alpha angle (α), which indicates the speed of clot strengthening (standard range, 53–72°). Clot stability is represented by the maximum amplitude (MA), which varies with the strength of the fibrin-platelet interaction (standard range, 50–70 mm). Lastly, lysis at 30 min (LY30) represents fibrinolysis (standard range, 0–8%) [14].
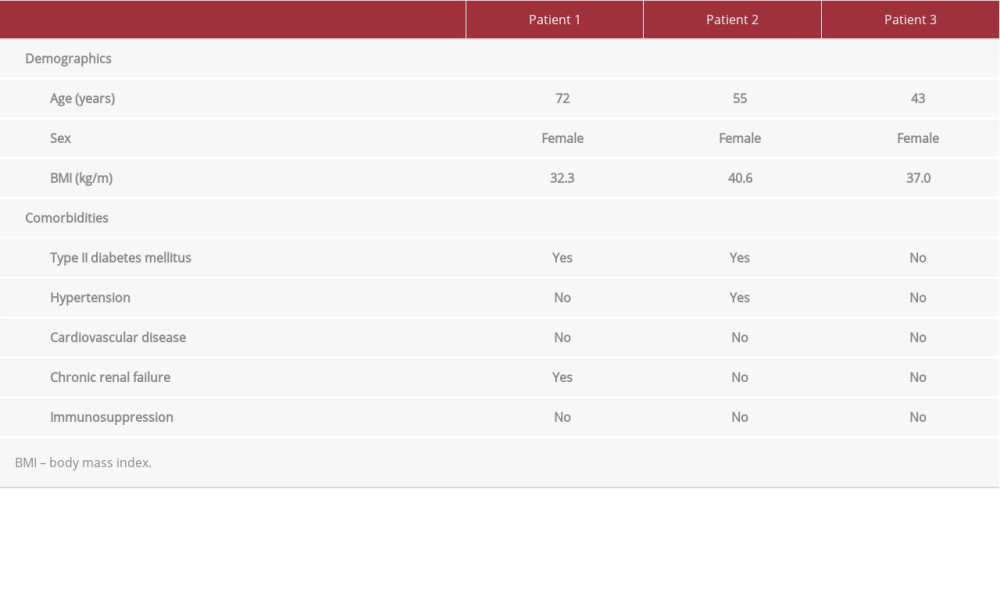
TEG/ROTEM have demonstrated utility in small studies to identify coagulopathies and manage anticoagulation in patients with COVID-19 [15–19]. TEG was selected over ROTEM in our study because of the hospital laboratory’s preexisting familiarity in application to trauma and surgery. All 3 cases presented below were confirmed positive for COVID-19 by reverse transcriptase-polymerase chain reaction assay of nasopharyngeal swabs, based on the World Health Organization standard that targets the SARS-CoV-2 E gene and RdRp gene (BioFire Respiratory 2.1 Panel, BioFire Diagnostics, Salt Lake City, UT, USA) [20,21]. Here, the utility of TEG (TEG 5000 analyzer with reagent kaolin and without heparinase, Haemonetics Corporation, Niles, IL, USA) as an adjunct to aPTT (Sysmex CA-1500 with reagents Innovin and CaCl2, Siemens Medical Solutions, Malvern, PA, USA) is demonstrated for 3 patients hospitalized with COVID-19-associated coagulopathy to enable personalized anticoagulant therapy. The patients’ demographic data are shown in Table 1.
Case Reports
CASE 1:
A 72-year-old woman on long-term warfarin therapy for a history of VTEs presented with COVID-19 pneumonia. Three days after admission, she was diagnosed with a right upper lobe PE, despite an international normalized ratio (INR) of 6.4 and aPTT of 120.7 s (reference range, 23.0–34.0 s) (Figure 1B). Intravenous (IV) UFH of 8 units/kg/h was started. Doppler ultrasound of the upper and lower extremities did not reveal a deep vein thrombus (DVT). The following day, TEG demonstrated severe hypocoagulability (Figure 1A). The UFH dosage was decreased to 3 units/ kg/h. For the next 2 weeks, TEG enabled goal-directed therapy with small doses of UFH from 3 to 5 units/kg/h, the goal being maintenance of the TEG parameter R of 12 to 17 min and aPTT of 50 to 60 s. It took the duration of these 2 weeks for the INR to decrease to subtherapeutic levels. The patient’s right upper lobe pulmonary infarct was no longer present on repeat imaging 14 days after its discovery (Figure 1C). On the same day as repeat imaging, TEG demonstrated normal hemostasis, and her aPTT was 25.0 s (Figure 1A). The patient was placed on apixaban 5 mg twice daily. In this case, there was no attempt to diagnose lupus anticoagulant or acquired hemophilia A because the severe prolongation of aPTT returned to normal 3 days after withholding warfarin therapy. Subsequently, heparin was used to provide anticoagulant therapy guided by TEG.
CASE 2:
A 55-year-old woman with moderately severe COVID-19 pneumonia was placed on therapeutic anticoagulation with 100 mg subcutaneous (SQ) enoxaparin every 12 h upon admission to the ICU for worsening respiratory insufficiency. Two days after admission, she developed a spontaneous rectus sheath hematoma, and the enoxaparin was held (Figure 2B). Four days after admission, TEG and aPTT demonstrated hypercoagulability, and she was restarted on anticoagulants. IV UFH was chosen over enoxaparin to prevent SQ administration in the abdomen where the rectus sheath hematoma was present. In addition, anti-factor Xa levels were not accessible to monitor enoxaparin. A Doppler ultrasound of the patient’s extremities was negative for DVT. For the next 8 days, UFH dosing from 8 to 12 units/kg/h was guided by the TEG to maintain a target aPTT of 40 to 50 s. Twelve days after admission, TEG revealed a LY30 of 28.5%, indicating fibrinolysis (Figure 2A). Concern for an expanding hematoma prompted a decrease in the UFH dosage from 8 to 7 units/kg/h. Fifteen days after admission, repeat imaging of the abdomen revealed a marginal decrease in size of the rectus sheath hematoma (Figure 2C). For the next 4 days, the patient was maintained on 7 units/kg/h of IV UFH. Seventeen days after admission, her treatment with IV heparin was stopped in preparation for discharge, and TEG demonstrated hemostasis (Figure 2A).
CASE 3:
A 43-year-old woman with no past medical history was transferred with moderately severe COVID-19 pneumonia, hypoxia, bilateral edema of the lower extremities, bilateral submassive pulmonary emboli, and acute-onset idiopathic thrombocytopenic purpura, diagnosed serologically. A Doppler ultrasound of all 4 extremities revealed a right popliteal DVT. She had a platelet count of 37×109/L (reference range, 130–470×109/L) and fibrinogen level of 137 mg/dL (reference range, 180–350 mg/dL) on admission. In addition, her D-dimer level was beyond the upper limit of the laboratory measurement of 35.2 mg/L (reference range, <0.53 mg/L). She was administered fibrinogen concentrate and platelets periodically to maintain a platelet count >50×109/L and fibrinogen >150 mg/dL. She also received a dose of romiplostim (Nplate) to stimulate thrombopoiesis. Subsequent testing for heparin-induced thrombocytopenia by serotonin release assay and platelet-Factor 4 antibody serology was negative. For the next 7 days, owing to concern for thrombocytopenia, hypofibrinogenemia, and an elevated D-dimer level, she was administered IV UFH doses of 15 to 20 units/kg/h to initially maintain a goal aPTT of 40 to 50 s. The goal was to prevent hemorrhagic manifestation of the thrombohemorrhagic pheno-type while simultaneously escalating anticoagulation therapy. Seven days after admission, computed tomography revealed the development of renal vein thrombosis, inferior vena cava thrombosis, and right external and internal iliac thromboses, despite high dose anticoagulation (Figure 3B). With significant thrombus burden, a higher aPTT goal of 60 to 70 s was targeted, despite a TEG tracing that was nearly a flat line (Figure 3A).
Repeat abdominal imaging conducted 18 days after admission revealed enlargement of venous thrombi (Figure 3C). A decision was made to further increase the dosage of UFH to achieve a target aPTT of 70 to 80 s, resulting in a severely hypocoagulopathic TEG showing a complete flat line (Figure 3A). After this final escalation of UFH, a gradual reduction of the D-dimer level and increase in fibrinogen occurred under this regimen for the next 10 days. The patient’s hypoxia, dyspnea, and lower limb edema resolved prior to discharge. Repeat imaging to demonstrate clot resolution prior to discharge was not obtained because the patient was asymptomatic, and the family requested no further testing owing to finances. Subsequent follow-up revealed that the patient had returned to normal occupational function and had no residual pulmonary or vascular symptoms.
Discussion
These 3 cases demonstrate the utility of TEG in guiding anticoagulation therapy for complex and coagulopathic critically ill patients with COVID-19 in the ICU. Case 1 represents the utility of TEG to guide heparin in a patient where a warfarin-induced INR of 6.4 did not prevent the development of a PE associated with COVID-19 pneumonia. Low doses of UFH were administered with the notion that the anti-inflammatory and antiviral effects of UFH would assist in preventing further expansion of the thrombus in the lung [22]. The pulmonary ‘embolus’ in this case may be a misnomer since the patient had no VTE on 4-extremity ultrasound. Rather, this was likely a pulmonary immunothrombus caused by the local endothelial inflammation in the pulmonary vasculature [2,6,23]. The resolution of the soft clot after 2 weeks of UFH therapy may not only have been due to the direct anticoagulation, but also indirectly due to the anti-inflammatory and antiviral effects of the heparin molecule at the endothelial surface [22].
In Case 2, TEG unveiled fibrinolysis otherwise undetectable by common coagulation tests, enabling the personalized adjustment of anticoagulation. It is uncertain whether the excessive fibrinolysis was causative of the hematoma or was a secondary response to the presence of the hematoma. Severely ill patients with COVID-19 more commonly demonstrate fibrinolytic shutdown, so this TEG pattern should prompt a search for an occult hemorrhage such as in this patient [15,19,24]. Finally, Case 3 demonstrates the immunothrombotic phenotype common in critically ill patients with COVID-19, who may clot despite therapeutic levels of anticoagulation [2,6,7]. Informed by the patient’s clinical state and assisted by TEG, the aPTT goal was escalated gradually for this patient with significant thrombus burden, which resulted in complete recovery.
The COVID-19 coagulopathy is dependent on the unique immunothrombotic changes that occur at the endothelium, which can cause an endotheliitis [23]. Although this more selectively involves the lung, it can potentially involve any vascular tissue. As there is infiltration of monocytes and macrophages into involved sites, an acute inflammatory response is induced with elevated levels of proinflammatory cytokines such as inter-leukin (IL)-6, IL-1, tumor necrosis factor α, and IL-8 [25,26]. In severe cases, the response is to a degree that is described as a “cytokine storm”. These inflammatory cytokines in turn activate the intrinsic pathway of the coagulation cascade, with factor XII activation and progression to thrombin generation. Fibrinolysis is also impaired with upregulation of plasminogen activator inhibitor 1 expression [27].
The effect of this cytokine storm on the endothelial surface may cause a pathophysiologic milieu like that found in patients on ECMO, as well as in patients who are post-resuscitative and recovering from trauma [11,28]. The persistence of fibrinolytic shutdown in all patients with COVID-19, regardless of whether the patient bled, reflects one of the interesting pathophysio-logic moments that defines the hypercoagulopathy of this novel disease [19,24]. The intensity of required anticoagulation in these patients may be a function of the intensity of inflammation caused by the cytokine storm. As the storm abates, so too does the requirement of higher levels of anticoagulation, suggesting the need for intense hemostatic monitoring not just with the aPTT, but also with adjunctive TEG analysis. We have drawn from the experience of ECMO, whereby frequent monitoring by a specialized coagulation team follows the clinical pattern of aPTT, anti-factor Xa levels, and TEG to guide anticoagulation therapy [11]. For patients with COVID-19, we utilized the same strategy by following biochemical inflammatory markers, D-dimer, fibrinogen, aPTT, and TEG to guide anticoagulation. Notably, we were unable to use anti-factor Xa levels owing to limitations at our institution, but in an ideal situation anti-factor Xa levels would also be used to adjudicate UFH therapy. We have found this strategy helpful for our patients, as demonstrated by the 3 examples described above of goal-directed and personalized anticoagulation for patients with COVID-19-induced coagulopathy.
Conclusions
These 3 cases of COVID-19 pneumonia with complex and varied clinical histories demonstrated the value of using TEG combined with the measurement of aPTT to facilitate individualized anticoagulation, resulting in good clinical outcomes.
Figures
References:
1.. : Coronavirus Disease 2019 (COVID-19) treatment guidelines, 2020, National Institutes of Health [cited 14 Feb 2021]. https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/
2.. Chowdhury JF, Moores LK, Connors JM, Anticoagulation in hospitalized patients with COVID-19: N Engl J Med, 2020; 383(17); 1675-78
3.. Boscolo A, Spiezia L, Correale C, Different hypercoagulable profiles in patients with COVID-19 admitted to the internal medicine ward and the intensive care unit: Thromb Haemost, 2020; 120(10); 1474-77
4.. Almskog L, Wikman A, Svensson J, Rotational thromboelastometry predicts care level in Covid-19: J Thromb Thrombolysis, 2020; 51(2); 437-45
5.. Al-Samkari H, Karp Leaf RS, Dzik WH, COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection: Blood, 2020; 136(4); 489-500
6.. Desborough MJ, Doyle AJ, Griffiths A, Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom: Thromb Res, 2020; 193; 1-4
7.. Fraissé M, Logre E, Pajot O, Thrombotic and hemorrhagic events in critically ill COVID-19 patients: A French monocenter retrospective study: Crit Care, 2020; 24(1); 275
8.. Musoke N, Lo KB, Albano J, Anticoagulation and bleeding risk in patients with COVID-19: Thromb Res, 2020; 196; 227-30
9.. , NIH ACTIV Trial of blood thinners pauses enrollment of critically ill COVID-19 patients [press release], 2020 [cited 14 Feb 2021]. https://www.nih.gov/news-events/news-releasesnih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients
10.. Gonzalez E, Moore EE, Moore HB, Management of trauma-induced coagulopathy with thromboelastography: Crit Care Clin, 2017; 33(1); 119-34
11.. Colman E, Yin EB, Laine G, Evaluation of a heparin monitoring protocol for extracorporeal membrane oxygenation and review of the literature: J Thorac Dis, 2019; 11(8); 3325-35
12.. Akay OM, The double hazard of bleeding and thrombosis in hemostasis from a clinical point of view: A global assessment by rotational thromboelastometry (ROTEM): Clin Appl Thromb Hemost, 2018; 24(6); 850-58
13.. Whiting P, Al M, Westwood M, Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: A systematic review and cost-effectiveness analysis: Health Technol Assess, 2015; 19(58); 1-228
14.. Sakai T, Comparison between thromboelastography and thromboelastometry: Minerva Anestesiol, 2019; 85(12); 1346-56
15.. Collett LW, Gluck S, Strickland RM, Evaluation of coagulation status using viscoelastic testing in intensive care patients with coronavirus disease 2019 (COVID-19): An observational point prevalence cohort study: Aust Crit Care, 2020 [Online ahead of print]
16.. Panigada M, Bottino N, Tagliabue P, Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis: J Thromb Haemost, 2020; 18(7); 1738-42
17.. Yuriditsky E, Horowitz JM, Merchan C, Thromboelastography profiles of critically ill patients with coronavirus disease 2019: Crit Care Med, 2020; 48(9); 1319-26
18.. Roh DJ, Eiseman K, Kirsch H, Hypercoagulable viscoelastic blood clot characteristics in critically ill coronavirus disease 2019 patients and associations with thrombotic complications: J Trauma Acute Care Surg, 2021; 90(1); e7-12
19.. Kruse JM, Magomedov A, Kurreck A, Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis: Crit Care, 2020; 24(1); 676
20.. Corman VM, Landt O, Kaiser M, Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR: Euro Surveill, 2020; 25(3); 2000045
21.. Sethuraman N, Jeremiah SS, Ryo A, Interpreting diagnostic tests for SARSCoV-2: JAMA, 2020; 323(22); 2249-51
22.. Barrett CD, Moore HB, Yaffe MB, ISTH interim guidance on recognition and management of coagulopathy in COVID-19: A comment: J Thromb Haemost, 2020; 18(8); 2060-63
23.. Varge Z, Flammer AJ, Steiger P, Endothelial cell infection and endotheliitis in COVID-19: Lancet, 2020; 395(10234); 1417-18
24.. Wright FL, Vogler TO, Moore EE, Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection: J Am Coll Surg, 2020; 231(2); 193-203
25.. Ye Q, Wang B, Mao J, The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19: J Infect, 2020; 80; 607-13
26.. Soy M, Keser G, Atagunduz P, Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment: Clin Rheumatol, 2020; 39; 2085-94
27.. Bhandary YP, Shetty SK, Marudamuthu AS, Regulation of lung injury and fibrosis by p53-mediated changes in urokinase and plasminogen activator inhibitor-1: Am J Pathol, 2013; 183; 131-43
28.. Moore HB, Moore EE, Neal MD, Fibrinolysis shutdown in trauma: Historical review and clinical implications: Anesth Analg, 2019; 129(3); 762-73
Figures
In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250