14 September 2021: Articles 
Late-Onset Systemic Lupus Erythematosus Associated with Autoimmune Hemolytic Anemia and Sixth Cranial Nerve Palsy
Challenging differential diagnosis
Kaku Kuroda1AEF*, Masaki Itagane2AEF, Mitsuyo Kinjo2AEFDOI: 10.12659/AJCR.932959
Am J Case Rep 2021; 22:e932959
Abstract
BACKGROUND: Patients with late-onset systemic lupus erythematosus (SLE) do not present with typical SLE symptoms or serology, and this can lead to a major delay in diagnosis. We report a complex case of an older woman who developed autoimmune hemolytic anemia and sixth cranial nerve palsy that posed considerable challenges in diagnosing late-onset SLE.
CASE REPORT: A 78-year-old Japanese woman presented with polyarthritis associated with generalized fatigue for 2 months, who later developed diplopia. Physical examination revealed conjunctival pallor, polyarthritis, and subsequent development of sixth cranial nerve palsy. Laboratory data revealed a decreased white blood cell count; macrocytic anemia; elevated levels of lactate dehydrogenase, indirect bilirubin, and erythrocyte sedimentation rate; hypocomplementemia; positive Coombs test; antinuclear antibodies (ANAs, 1: 40); and positive anti-double-strand DNA antibodies. Lymphoma, cerebral venous sinus thrombosis, and varicella-zoster virus infection were unlikely based on head computed tomography, brain magnetic resonance imaging, and cerebrospinal fluid analysis. She was diagnosed with late-onset SLE associated with autoimmune hemolytic anemia and sixth cranial nerve palsy. The patient was successfully treated with prednisone and hydroxychloroquine.
CONCLUSIONS: The difficulty in diagnosing late-onset SLE with atypical presentations and uncommon complications must be recognized. SLE cannot be excluded based on a low titer of ANA in a particular subgroup such as the elderly, and the prozone effect should be considered responsible for low ANA titers. In this case, late-onset SLE was diagnosed by considering multisystem pathologies despite low ANA titers.
Keywords: Abducens Nerve Diseases, Anemia, Hemolytic, Autoimmune, Lupus Erythematosus, Systemic, Antibodies, Antinuclear, Female, Humans, hydroxychloroquine
Background
Systemic lupus erythematosus (SLE) is an autoimmune disorder that most commonly affects young women [1]. However, in some cases it develops later in life after 50 years of age; this is referred to as late-onset SLE [2]. Such patients do not present with typical SLE symptoms or serology [3], which can lead to a major delay in diagnosis. In the current case, the presence of autoimmune hemolytic anemia (AIHA) and sixth cranial nerve palsy in an older patient posed considerable challenges in diagnosing late-onset SLE.
Case Report
A 78-year-old Japanese woman presented to the Emergency Department with polyarthritis and generalized fatigue. The patient reported a 2-month history of joint pain in her bilateral wrists, knees, and ankles, which was associated with generalized fatigue and became immobile. She denied fever, chills, weight loss, night sweats, cough, or gastrointestinal symptoms. Three months ago, she developed a painful tingling sensation and multiple small vesicles on her back. Subsequently, she was diagnosed with herpes zoster at a local clinic. Two months prior to presentation to our facility, she developed multiple joint pain. Her medical history was significant for an anemia of unknown etiology 5 years ago, for which she had received prednisolone for 2 years at a different hospital. Her medications included acetaminophen and duloxetine for post-herpetic neuralgia. The patient owned a hotel, lived by herself, and denied tobacco smoking, alcohol, and illicit drug use. There was no relevant family history. Her vital signs were within normal limits. Her physical examination revealed conjunctival pallor; bilateral tenderness and swelling of the wrist, knee, and ankle joints; and pitting edema of both lower legs.
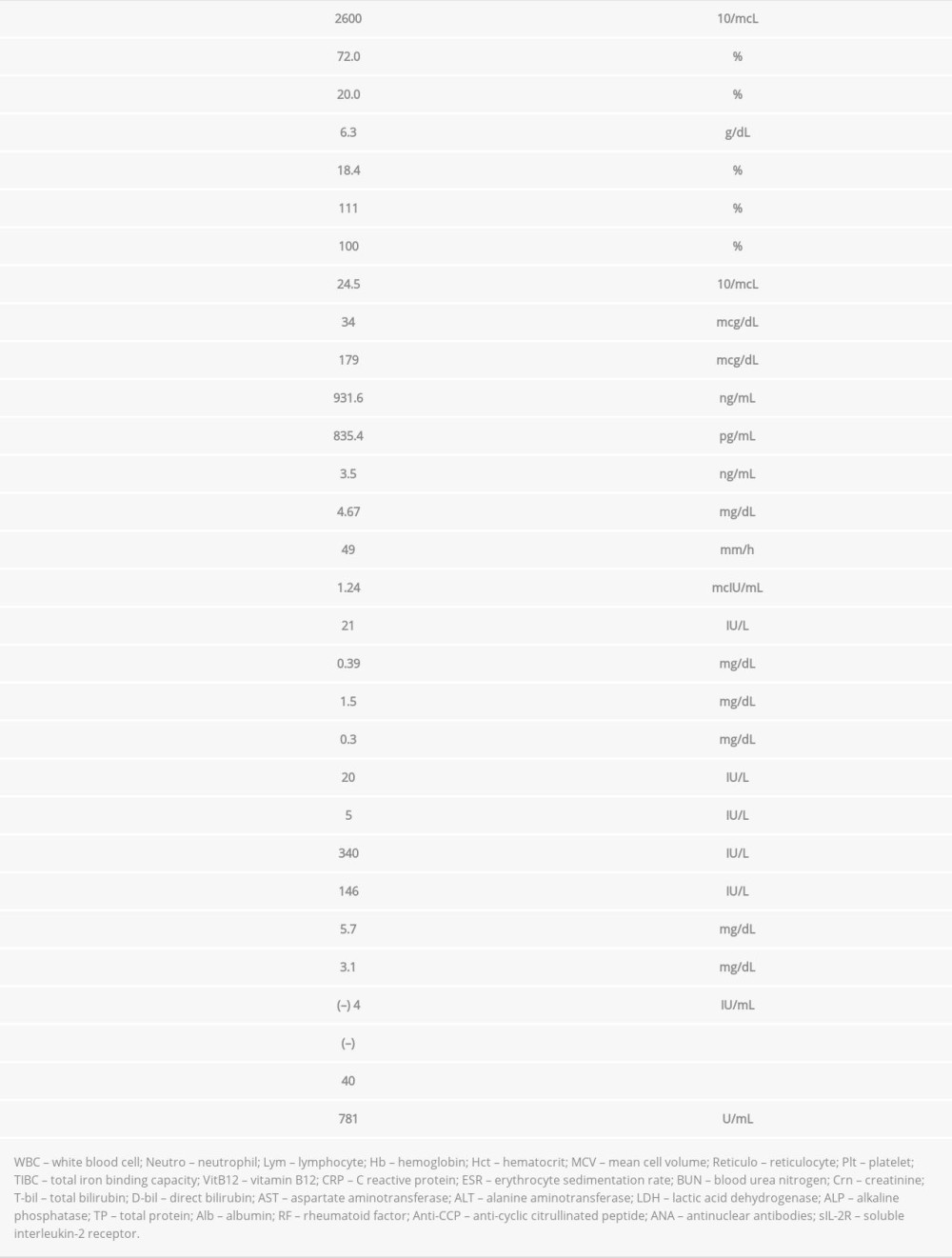
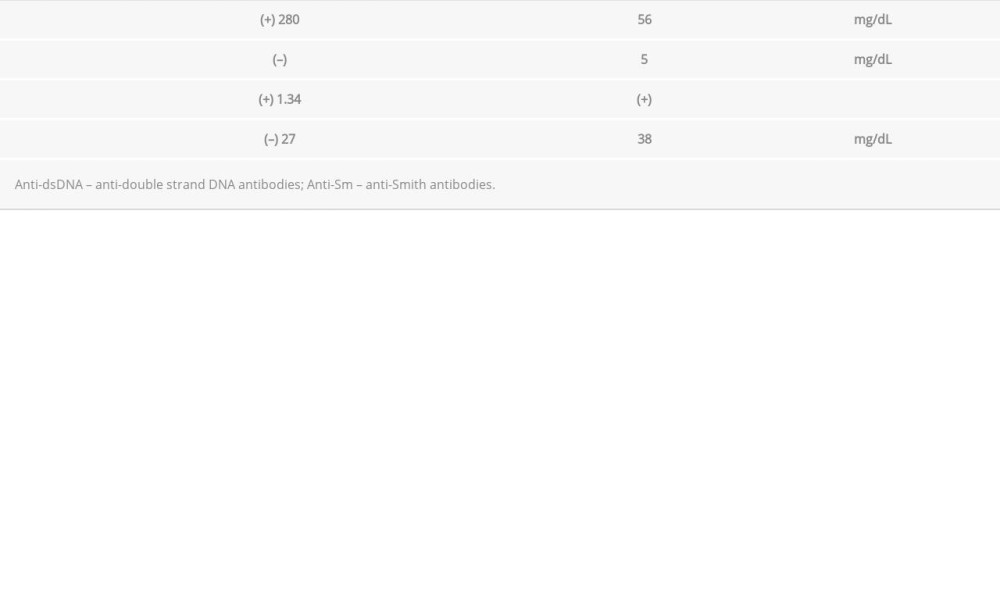
Laboratory data were as follows: white blood cell count, 2500/mcl; macrocytic anemia (hemoglobin, 7.2 g/dL; hematocrit, 23.6%; mean corpuscular volume, 106.3 fl with normal vitamin B12 and folate levels); total bilirubin, 1.5 mg/dL; direct bilirubin, 0.3 mg/dL; lactate dehydrogenase, 426 IU/L; and erythrocyte sedimentation rate, 113 mm/h (Table 1). Hemolytic anemia was suspected, and the patient was scheduled for follow-up after 1 week. Two days after the initial visit, she developed diplopia and became immobile owing to worsening joint pain. At the follow-up visit 1 week after the initial visit, her body temperature was 37.9°C (100.2°F). She had no other symptoms, except for the development of left sixth cranial nerve palsy (Figure 1). A Coombs test for AIHA was positive. The new onset of isolated left sixth cranial nerve palsy and hemolytic anemia prompted physicians to suspect underlying hematological or autoimmune conditions such as lymphoma, myelodysplastic syndrome, SLE, or protracted varicella-zoster virus infection. Additional blood test results revealed hypocomplementemia, antinuclear antibodies (ANAs) 1: 40 with a homogenous pattern, negative rheumatoid factor and anti-cyclic citrullinated peptide antibodies, positive anti-double-stranded DNA antibodies as confirmed with the Crithidia luciliae method, positive lupus anticoagulant, and a biological false positive for rapid plasma reagin (Table 2).
Given the left sixth cranial nerve palsy, a cerebrospinal fluid analysis was conducted, which was negative for varicella-zoster virus antibodies (IgM and IgG). Brain computed tomography and magnetic resonance imaging revealed old cerebral infarcts but no evidence of tumor, metastasis, or cerebral venous sinus thrombosis. The ophthalmologic evaluation was normal. After obtaining a detailed history from the patient’s previous hospital, it was found that her ANAs were negative 5 years ago. In view of the Coombs-positive hemolytic anemia and a positive serology for lupus, the sixth cranial nerve palsy was attributed to SLE.
Administration of oral prednisone 0.5 mg/kg/day (25 mg daily) was initiated for AIHA associated with late-onset SLE. We did not add rituximab to the initial glucocorticoid because the patient had responded well to glucocorticoid, and we focused on avoiding adverse effects given her old age. Fever, arthralgia, and sixth cranial nerve palsy gradually resolved, and her hemoglobin level returned to baseline within 2 weeks. Hydroxychloroquine 200 mg/day was added. At follow-up 18 months after her initial visit, she remained asymptomatic.
Discussion
We report a complicated case of an older woman who developed AIHA and sixth cranial nerve palsy. Advanced age, low titers of positive ANA, AIHA, and isolated sixth cranial nerve palsy at the outset made the diagnosis of SLE challenging.
Late-onset SLE that develops in an advanced age has been increasingly reported, affecting 12–18% of all patients with SLE [4]. Late-onset SLE is reported to have a milder disease course than SLE in the population aged 49 years or younger [5]. Clinical manifestations have different features such as less frequent arthritis [6–8], malar rash [6–9], and lesser photosensitivity [7–9]. In laboratory tests, the prevalence of hemolytic anemia and thrombocytopenia in late-onset SLE is inconsistent [6,8–10]. In late-onset SLE, low complement levels are uncommon [8,11,12], and the anti-ds DNA antibody level may be lower in some studies [6,10–12]. In the present case, arthritis, AIHA, leukocytopenia, hypocomplementemia, anti-ds DNA antibody, and lupus anticoagulant had significantly increased and were consistent with the clinical picture of SLE.
Regarding ANA (1: 40), we have 2 insights for this finding being inconsistent with SLE in the present case. First, SLE cannot be excluded based on a low titer of ANA in a particular subgroup [13–15]. Studies showed that a low ANA titer is more commonly observed in older patients [16]. Second, the pro-zone effect should be considered a cause of low ANA titers with a clinical picture and laboratory data consistent with SLE. Highly positive anti-ds DNA antibody may block the antigen-antibody reaction, resulting in a false negative or low ANA titer, the “prozone effect”, in late-onset SLE [17]. In our case, since the patient met both the ACR and SLICC criteria [18,19], we believe that a low ANA titer did not rule out the diagnosis of late-onset SLE.
AIHA in older patients may be associated with various underlying diseases, which often makes it challenging to diagnose SLE, particularly when other clinical features are not evident during initial disease presentation. In patients with SLE, AIHA is one of the most common etiologies of anemia [20]. It is observed in up to 10% of patients with SLE across all ages [21,22]. However, reports on the age distribution of AIHA in SLE are conflicting. In older patients diagnosed with AIHA, it is reported that AIHA is most frequently associated with lymphoproliferative disorders [23,24], followed by autoimmune conditions, especially SLE [24]. Repeated episodes of AIHA should be considered in association with late-onset SLE when the underlying hematological disease is not evident.
Only a few cases of isolated sixth cranial nerve palsy have been reported as a manifestation of SLE [25,26]. In this case, other conditions potentially associated with sixth cranial nerve palsy, including lymphoma, meningitis, Guillain-Barre syndrome, varicella-zoster virus, multiple sclerosis, cerebral venous sinus thrombosis, and diabetes mellitus, were excluded. Moreover, the temporality of arthritis and AIHA with sixth cranial nerve palsy supported the diagnosis of SLE. Similar to a previous report, complete resolution of symptoms was observed within 2 weeks of steroid therapy, based on which the underlying pathogenesis of thrombotic vasculopathy in a positive lupus anticoagulant profile could be considered [26].
Conclusions
This case allowed us to learn that patients with late-onset SLE may not present with typical SLE symptoms or serology. We need to recognize the difficulty in diagnosing late-onset SLE with atypical presentations and even with uncommon complications such as AIHA and cranial nerve palsy to provide timely and appropriate treatment. Although the new European League Against Rheumatism/American College of Rheumatology classification criteria for SLE suggests using ANA as an entry criterion, SLE is still diagnosed with low ANA titers and characteristic manifestations with positive autoimmune antibodies such as anti-ds DNA antibody. Finally, the diagnosis of late-onset SLE is achieved by comprehensively considering disease groups involving multisystemic pathologies such as fever and pathologies of the joints, blood, and nerves.
References:
1.. Tsokos GC, Systemic lupus erythematosus: N Engl J Med, 2011; 365; 2110-21
2.. Boddaert J, Huong DLT, Amoura Z, Late-onset systemic lupus erythematosus: A personal series of 47 patients and pooled analysis of 714 cases in the literature: Medicine (Baltimore), 2004; 83; 348-59
3.. Rovenský J, Tuchyňová A, Systemic lupus erythematosus in the elderly: Autoimmun Rev, 2008; 7; 235-39
4.. Aljohani R, Gladman DD, Su J, Urowitz MB, Disease evolution in late-onset and early-onset systemic lupus erythematosus: Lupus, 2017; 26; 1190-96
5.. Joo YB, Park SY, Won S, Bae SC, Differences in clinical features and mortality between childhood-onset and adult-onset systemic lupus erythematosus: A prospective single-center study: J Rheumatol, 2016; 43; 1490-97
6.. Appenzeller S, Pereira DA, Costallat LT, Greater accrual damage in late-onset systemic lupus erythematosus: A long-term follow-up study: Lupus, 2008; 17; 1023-28
7.. Cervera R, Khamashta MA, Font J, Systemic lupus erythematosus: Clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus: Medicine (Baltimore), 1993; 72; 113-24
8.. Boddaert J, Huong DL, Amoura Z, Late-onset systemic lupus erythematosus: A personal series of 47 patients and pooled analysis of 714 cases in the literature: Medicine (Baltimore), 2004; 83; 348-59
9.. Tomic-Lucic A, Petrovic R, Radak-Perovic M, Late-onset systemic lupus erythematosus: clinical features, course, and prognosis: Clin Rheumatol, 2013; 32; 1053-58
10.. Catoggio LJ, Soriano ER, Imamura PM, Late-onset systemic lupus erythematosus in Latin Americans: A distinct subgroup?: Lupus, 2015; 24; 788-95
11.. Sohn IW, Joo YB, Won S, Bae SC, Late-onset systemic lupus erythematosus: Is it “mild lupus”?: Lupus, 2018; 27; 235-42
12.. Choi JH, Park DJ, Kang JH, Comparison of clinical and serological differences among juvenile-, adult-, and late-onset systemic lupus erythematosus in Korean patients: Lupus, 2015; 24; 1342-49
13.. Aringer M, Costenbader K, Daikh D, 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus: Arthritis Rheumatol, 2019; 71; 1400-12
14.. Suda M, Kishimoto M, Ohde S, Okada M, Validation of the 2019 ACR/EULAR classification criteria of systemic lupus erythematosus in 100 Japanese patients: A real-world setting analysis: Clin Rheumatol, 2020; 39; 1823-27
15.. Anyfantakis D, Symvoulakis EK, Barbounakis E, A fatal case of sero-negative, late-onset systemic lupus erythematosus presenting with motor sensory axonal polyneuropathy: Mod Rheumatol, 2014; 24; 858-61
16.. Choi MY, Clarke AE, St Pierre Y, Antinuclear antibody-negative systemic lupus erythematosus in an international inception cohort: Arthritis Care Res (Hoboken), 2019; 71; 893-902
17.. Jin J, Zehnder JL, Prozone effect in the diagnosis of lupus anticoagulant for the lupus anticoagulant-hypoprothrombinemia syndrome: Am J Clin Pathol, 2016; 146; 262-67
18.. Hochberg MC, Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus: Arthritis Rheum, 1997; 40; 1725
19.. Petri M, Orbai AM, Alarcón GS, Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus: Arthritis Rheum, 2012; 64; 2677-86
20.. Fayyaz A, Igoe A, Kurien BT, Hematological manifestations of lupus: Lupus Sci Med, 2015; 2; e000078
21.. Giannouli S, Voulgarelis M, Ziakas PD, Tzioufas AG, Anemia in systemic lupus erythematosus: from pathophysiology to clinical assessment: Ann Rheum Dis, 2006; 65; 144-48
22.. Keeling DM, Isenberg DA, Hematological manifestations of systemic lupus erythematosus: Blood Rev, 1993; 7; 199-207
23.. Zulfiqar AA, Pennaforte JL, Andres EJ, Autoimmune hemolytic anemia in individuals aged 75 and older: A study of 10 individuals: J Am Geriatr Soc, 2016; 64; 1372-74
24.. Roumier M, Loustau V, Guillaud C, Characteristics and outcome of warm autoimmune hemolytic anemia in adults: New insights based on a single-center experience with 60 patients: Am J Hematol, 2014; 89; E150-55
25.. Sedwick LA, Burde RM, Isolated sixth nerve palsy as initial manifestation of systemic lupus erythematosus: J Clin Neuroophthalmol, 1983; 3; 109-10
26.. Saleh Z, Menassa J, Abbas O, Cranial nerve VI palsy as a rare initial presentation of systemic lupus erythematosus: Case report and review of the literature: Lupus, 2010; 19; 201-5
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250