10 December 2021: Articles 
as a Cause of Pneumonia and Disseminated Infection in Febrile Neutropenia: A Case Report and Literature Review
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Rare disease
Batool M. Abu Ali1CDEF*, Hibah Alzayer2DF, Marwan Jabr Alwazzeh1D, Asim Diab2DDOI: 10.12659/AJCR.933694
Am J Case Rep 2021; 22:e933694
Abstract
BACKGROUND: Prototheca spp. are common and found in various environments, including animal and human intestines, on the skin and in respiratory tissues, and colonizing fingernails. Few strains pathogenic for humans have been discovered. Here, we describe an infection by the pathogenic fungus species Prototheca zopfii in a patient. The infection was initially classified as a fungus based on colony morphology, fungal staining results, and growth in some fungi culture media (Sabouraud dextrose agar [SDA]). Reports of Prototheca spp. infections are increasing, often with poor outcomes. The use of matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) technique for identification has been widely described. Phenotypic identification depends on microscopic examination of the direct wet mount and after subculturing in blood and SDA using different stains that show a typical morphological characteristic of Prototheca spp.
CASE REPORT: A 48-year-old woman was diagnosed with a P. zopfii infection after 22 days of hospitalization in the critical care unit. The patient had profound febrile neutropenia and absolute neutrophil count (ANC) was zero, associated with hypotension and disseminated intravascular coagulation (DIC) 10 days after receiving the first cycle of chemotherapy for metastatic breast adenocarcinoma. Unfortunately, the patient died within 2 days of the initiation of treatment with amphotericin B.
CONCLUSIONS: This case report highlights algae infections as a possible opportunistic infection type in patients with profound neutropenia, and we discuss the use of MALDI-TOF MS-based technology in detecting such infections and predicting poor prognosis, especially in patients with the disseminated form with underlying febrile neutropenia.
Keywords: febrile neutropenia, Prototheca, protothecosis, amphotericin B, Animals, Female, Humans, Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
Background
A better understanding of this microorganism is needed because advances in chemotherapy and immune suppression therapy are increasing the burden of this disease. In this report, we describe the first reported case of disseminated
Case Report
We report a case of a 48-year-old single, unemployed woman, originally from Jazan (southern Saudi Arabia). The patient known to have diabetes mellitus type II, hypothyroidism because of total thyroidectomy due to thyroid cancer 10 years ago, and right breast adenocarcinoma (diagnosed and managed in another specialized hospital) with spine metastases complicated by paraplegia. She presented on 8 April 2021, 10 days after receiving the first cycle of chemotherapy (docetaxel) and 5 previous cycles of radiotherapy, with fever reaching 38.6°C, vomiting (grade 2–3), and watery diarrhea (grade 4), associated with fatigue and dizziness (grade 3) evaluated according to NCI CTCAE (National Cancer Institute common terminology criteria for adverse events). She reported having no history of hematemesis, melena, hematochezia, abdominal pain, herbal exposure, recent travel, or contact with domestic animals.
On physical examination in the Emergency Department, the patient was pale, hypotensive (blood pressure of 89/59 mmHg) and tachycardic (heart rate of 129 beats per min); body temperature was 38.6°C; respiratory rate was 22 breaths/min and oxygen saturation, and oxygen saturation was 97% on room air. She was conscious, alert, and oriented to time, place, and person. Results of an examination of the chest and abdomen were unremarkable. She had a clean sacral pressure ulcer grade 2 measuring 2.5×1.5 cm in size. After fluid resuscitation, her blood pressure improved.
Laboratory investigations on admission revealed pancytopenia, with a white blood cell (WBC) count of 0.2 k/µl and absolute neutrophil count (ANC) of 0 cell/µl; hemoglobin level was 8.9 g/L and platelet count was 7000/µl. The serum creatinine value was 1.1 mg/dl and blood urea nitrogen was 24 mg/dl. Estimation of electrolytes showed sodium 138 mEq/L, potassium 3.2 mEq/L, and bicarbonate 25 mmol/L. Urine analysis was normal and liver function tests were as follow: protein 5 g/dL, albumin 3.4 g/dL, total bilirubin 1.2 mg/dl, alanine transaminase 49 IU/L, aspartate transaminase 33 IU/L, alkaline phosphatase 179 IU/L, and gamma-glutamyl transferase 447 IU/L. Her lactate level was 1.8 mmol/L, procalcitonin was 138 ng/ml, and C-reactive protein was 36 mg/L. The patient had a clinical picture of DIC with prolonged thromboplastin time PT (47.3 s) and partial thromboplastin time PPT (49 s), high international normalized ratio INR (3.4), fibrinogen level 290 mg/dl and elevated D-dimer (3.0 µg/ml). Peripheral blood film shows normocytic normochromic anemia with mild anisocytosis, mild poikilocytosis, and few schistocytes. A chest X-ray (CXR) on admission (Figure 1) showed a normal lung field, clear bilaterally. She was started empirically on vancomycin (1 g i.v. every 12 h) and meropenem (1 g i.v. every 8 h).
Blood culture at presentation was positive for
Methicillin-resistant
On 13 April, the patient developed upper gastrointestinal (GI) bleeding followed by lower GI bleeding with fresh blood per rectum and was evaluated by General Surgery. She underwent proctoscopy, which showed normal mucosa with the presence of fresh blood. She received multiple blood products. The Gastroenterology team was consulted, and because of hemo-dynamic instability, gastroscopy and colonoscopy were delayed by a few days, and when performed, the delayed gastroscopy was unremarkable; however, the colonoscopy showed severe, inflamed, ulcerative lesions throughout the whole colon. Biopsies revealed inflamed fibromuscular stroma, ulcerative surface, and reactive atypia without malignancy, and no evidence of
On 19 April, total parenteral nutrition (TPN) was initiated through the right brachial by a peripherally-inserted central catheter (PICC) line. On 27April, the antifungal coverage shifted to caspofungin (70 mg i.v. loading dose, then 50 mg i.v. every 24 h).
On 30 April, she had respiratory deterioration, requiring intubation and mechanical ventilation (Figure 2). The anteroposterior (AP) view CXR showed patchy infiltration involving the upper left radiological zone, and multiple patches over the entire right lung field, as well as middle and lower left radiological zone, obliterating the left costophrenic angle (Figure 3). On post-intubation day 2, we detected severe bilateral pneumonia involving the middle and lower zone of the left lung and right upper zone. Tracheal aspirate and blood culture on 30 April were positive for
Discussion
Disseminated protothecosis is a fatal opportunistic infection that can arise in immunodeficient populations [12]. The pathogenesis of
Disseminated protothecosis may be clinically unsuspected and underdiagnosed; thus, patients may receive various treatments for extended periods with no satisfactory results [1]. The definitive diagnosis of infection often depends on the morphological identification of organisms in the preparation of the wet culture slide and/or direct identification in tissue samples [1–3].
Recently, differences in the protein expression profiles of environmental strains (genotype 1) and pathogenic strains (genotype 2) have been reported, suggesting that it is related to the level of
We typically identify microorganisms at the species level by use of MALDI-TOF MS. In this case, the isolate was identified using the manufacturer database that included a reference spectrum of
Treatment options are still controversial, and various treatments are being tried. Antifungal agents, such as ketoconazole, itraconazole, fluconazole, conventional amphotericin B, and liposomal amphotericin B, are the most commonly used drugs to date. Among them, amphotericin B showed the best activity against
Ultimately, we reported this case as disseminated protothecosis caused by
Conclusions
This case report shows that protothecosis is a serious infection type among immune-compromised patients and is associated with a high mortality rate and limited treatment options. It is increasingly common to combine an immune-suppressing agent with chemotherapy and steroid treatments. This case was diagnosed in our hospital based on laboratory identification, although there was an initial lack of suspicion. Early suspicion of such differential diagnoses would help to rapidly identify the best treatment. Food contaminated with
Figures
References:
1.. Lass-Flörl C, Mayr A, Human protothecosis: Clin Microbiol Rev, 2007; 20(2); 230-42
2.. Pore RS, Barnett EA, Barnes WC, Walker JD, Prototheca ecology: Mycopathologia, 1983; 81(1); 49-62
3.. Pore RS, Shahan TA: Mycopathologia, 1988; 101(2); 85-88
4.. Camboim EKA, Garino FJ, Dantas AFM: Mycoses, 2011; 54(4); e196-200
5.. Zhang Q, Weng X, Li L: Int J Infect Dis, 2010; 14; e32-35
6.. Todd JR, Matsumoto T, Ueno R, Medical phycology 2017: Med Mycol, 2018; 56(Suppl. 1); S188-204
7.. Hirose N, Hua Z, Kato Y: Med Mycol, 2018; 56(3); 279-87
8.. Todd JR, King JW, Oberle A, Protothecosis: Report of a case with 20-year follow-up, and review of previously published cases: Med Mycol, 2012; 50(7); 673-89
9.. Roesler U, Möller A, Hensel A: Int J Syst Evol Microbiol, 2006; 56(6); 1419-25
10.. Satoh K, Ooe K, Nagayama H, Makimura K: Int J Syst Evol Microbiol, 2010; 60(5); 1236-40
11.. Masuda M, Hirose N, Ishikawa T: Int J Syst Evol Microbiol, 2016; 66(3); 1510-20
12.. Sim JY, Hsueh PR, Chuang PC, Lee PI: J Microbiol Immunol Infect, 2019; 52(5); 833-35
13.. Freifeld AG, Bow EJ, Sepkowitz KA, Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America: Clin Infect Dis, 2011; 52(4); e56-93
14.. Takano M, Hoshi S, Nagai K: J Infect Chemother, 2014; 20(10); 647-49
15.. Milanov D, Suvajdzic L, Characteristics and importance of the genus Prototheca in human and veterinary medicine: Zb Matice Srp za Prir Nauk, 2006(111); 15-27
16.. Krcmery V, Systemic chlorellosis, an emerging infection in humans caused by algae: Int J Antimicrob Agents, 2000; 15(3); 235-37
17.. Kano R, Emergence of fungal-like organisms: Prototheca: Mycopathologia, 2020; 185(5); 747-54
18.. Sari S, Dalgic B, Muehlenbachs A: J Infect Dis, 2018; 218(3); 485-89
19.. Lass-Flörl C, Fille M, Gunsilius E: J Clin Microbiol, 2004; 42(10); 4907-8
Figures
Tables
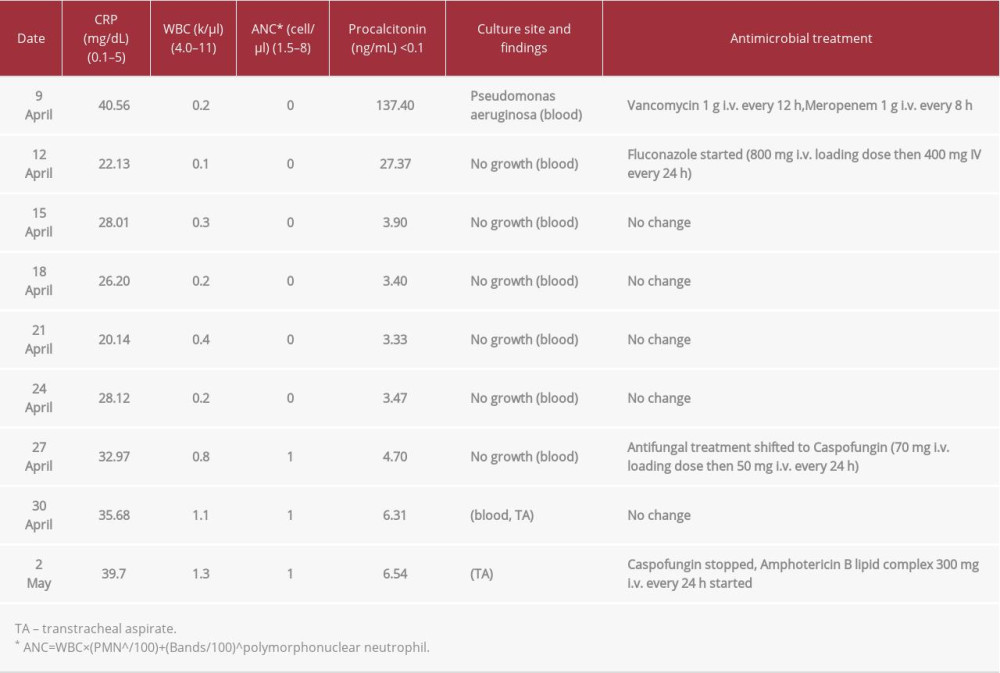 Table 1.. Important laboratory findings and antimicrobial management during ICU admission.
Table 1.. Important laboratory findings and antimicrobial management during ICU admission.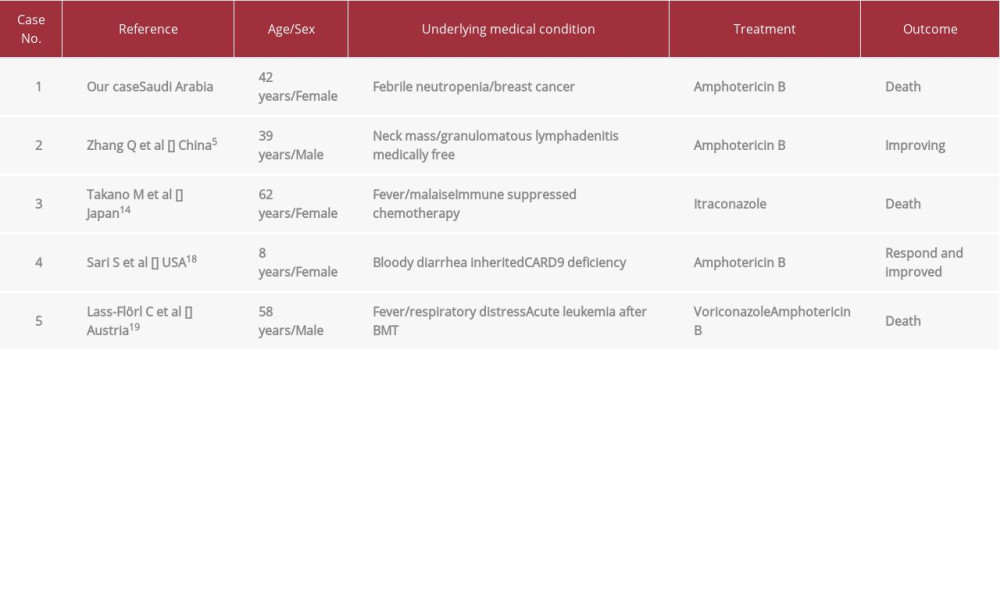 Table 2.. Documented disseminated Prototheca zopfii cases in the literature review.
Table 2.. Documented disseminated Prototheca zopfii cases in the literature review. Table 1.. Important laboratory findings and antimicrobial management during ICU admission.
Table 1.. Important laboratory findings and antimicrobial management during ICU admission. Table 2.. Documented disseminated Prototheca zopfii cases in the literature review.
Table 2.. Documented disseminated Prototheca zopfii cases in the literature review. In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








