14 February 2022: Articles 
Recurrent COVID-19 Polymerase Chain Reaction (PCR) Positivity in 2 Patients During the Current Health Care System Crisis
Challenging differential diagnosis, Diagnostic / therapeutic accidents, Educational Purpose (only if useful for a systematic review or synthesis)
Anna Radlińska1ADEFG*, Ewelina Drytkiewicz2BCD, Mateusz Patyk3DE, Anna Zaleska1AC, Anna Dor-Wojnarowska1CDFDOI: 10.12659/AJCR.935414
Am J Case Rep 2022; 23:e935414
Abstract
BACKGROUND: The coronavirus disease 2019 (COVID-19) pandemic is becoming challenging for public health crisis management. Effective detection method such as the criterion standard real-time reverse-transcription polymerase chain reaction (rRT-PCR) test is the only reliable option for the Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2). RT-PCR detects the genetic material of the virus but does not distinguish the infectious periods. Other diagnostic methods as serological tests and computed tomography (CT) are less accurate but can provide complementary information, especially in the face of new SARS-CoV-2 variants. Here, we report 2 cases of coronavirus-infected patients with recurrent RT-PCR positivity after recovery, which raised questions about possible reinfection.
CASE REPORT: A married couple, a 44-year-old woman and a 45-year-old man, after COVID-19 recovery, from April to August 2020 presented dynamic RT-PCR outcomes (oscillating from negative to positive). Anti-SARS-CoV-2 immunoglobulin G (IgG) levels for both patients were 1000 U/ml, indicating seroconversion. As a result of recurrent positivity, the patients were isolated and had limited access to healthcare. In the follow-up period, combining RT-PCR results with serology testing and CT allowed determination of the patients’ infectiousness.
CONCLUSIONS: Due to emerging coronavirus variants, individuals with dynamic PCR results, especially with post-COVID-19 syndrome, are indistinguishable from those who are infectious. Misdiagnosis causes unnecessary quarantines and exacerbates the health care crisis. Patients who had dynamic RT-PCR for SARS-CoV-2 require different diagnostics methods from those used in patients with a first-time positive test result. Combining diagnostic methods and identification of new variants of SARS-CoV-2 allows better estimation of the risk of reinfection.
Keywords: SARS-CoV-2 Variants, reinfection, Reverse Transcriptase Polymerase Chain Reaction, SARS-CoV-2, COVID-19 Diagnostic Testing, Adult, COVID-19, Delivery of Health Care, Female, Humans, Male, Real-Time Polymerase Chain Reaction
Background
Coronavirus disease 2019 caused by SARS-CoV-2 is currently one of the most significant public health concerns. According to the recommendations of the World Health Organisation (WHO) and the European Centre for Disease Prevention and Control (ECDC), the reference standard to confirm acute SARS-CoV-2 infection is laboratory-based molecular assays (nucleic acid amplification testing [NAAT]) detecting viral RNA in the respiratory secretions of the patient [1,2]. However, due to emerging coronavirus variants, interpreting rRT-PCR results, which is a common form of NAAT, can be challenging.
Here, we present an unusual case of 2 patients with dynamic rRT- PCR results (oscillating from negative to positive). We show the changing outcomes of molecular diagnosis in relation to serological assays and clinical symptoms. We also discuss the possibility of reinfection and interpretation of SARS-CoV-2 testing in relation to the current literature and knowledge.
Case Reports
CASE 1:
The 45-year-old man with a body mass index (BMI) of 33.24 kg/m2 and a history of chest trauma 4 years before, working outside the home in shifts, was quarantined in his apartment from April 4, 2020, due to his wife’s SARS-CoV-2 infection. On April 10, he presented COVID-19 symptoms of dry cough and loss of smell and taste. The first nucleic acid examination was inconclusive, until April 17th, when the man tested positive. For the entire duration of the disease, he was in good general condition and was released from home isolation when 2 negative PCR test results were obtained. Although after the recovery he reported extensive fatigue, he immediately returned to work.
Three months later, he was to be admitted to the Department of Internal Disease as a follow-up visit, but his nasopharyngeal swab for SARS-CoV-2 was positive (Viasure SARS-CoV-2 Real-Time PCR, CerTest Biotec, Spain). The test involved the amplification of a conserved region of ORF1ab and N genes for SARS-CoV-2 and had European CE-IVD (CE-marked in vitro diagnostic medical devices) approval for COVID-19 diagnostics [3]. The laboratory was participating in the EQA (External Quality Assessment) program. The man did not have any symptoms that could indicate an acute infection. He was not vaccinated, because none of the vaccines were available at the time. In June, he became a convalescent plasma donor. The anti-SARS-CoV-2 IgG level was 1000 U/ml (Roche Elecsys anti-SARS-CoV-2, Switzerland; the test sensitivity and specificity are 100% and 99.8%, respectively; the test is registered by the Food and Drug Administration [4]); nevertheless, because of fear of infectiousness, the emergency room operation was suspended for approximately 3 hours and the man was referred to home isolation for 14 days.
The patient was not admitted to the hospital until August, when a negative PCR test result was obtained (a timeline of individual symptoms in relation to rRT-PCR results and anti-SARS-CoV-2 antibodies is shown in Table 1). Then, the chest CT was performed. To reduce the radiation dose, we used automatic spectral imaging protocol selection (ASIS) combined with adaptive statistical iterative reconstruction (ASIR). The CT scans showed minor post-inflammatory changes: striking-like lesions, slightly reduced left lower-lobe aeration (Figure 1). There were no laboratory abnormalities.
The rate of anti-SARS-CoV-2-IgG was still 1000 U/ml (Roche, Switzerland). Three days after hospitalization, the man informed us that he was obliged to quarantine for 14 days due to close contact during his night shift with a person diagnosed with COVID-19.
CASE 2:
A previously healthy, 44-year-old, obese woman, on April 4, 2020, reported a dry cough, fever, anosmia, ageusia, and fatigue. On the eighth day of illness, she was admitted to the hospital presenting high fever and dyspnea. The chest X-ray showed bilateral lobar consolidations. rRT-PCR test was positive (ORF1 ab and the E gene assay, the swab sample was collected by qualified healthcare providers and the test was performed in an accredited laboratory for diagnostic RT-PCR testing in infectious diseases ward). The persistent low-flow oxygen support was needed. She was treated with intravenous antibiotics, dexamethasone, and chloroquine. The clinical improvement was obtained and the woman was discharged 21 days after admission, having 2 negative rRT-PCR results. After the recovery she still was unwell because of experienced post-Covid syndrome (Table 2). However, she was able to work from home during the national lockdown and from June to October she was on summer break. A follow-up chest CT was scheduled in mid-July to assess pulmonary damage, but the visit didn’t take place because RT-PCR for SARS-CoV-2 resulted inconclusive (all the tests were performed using the same methods and in the same laboratory as in case 1). On the same day SARS-CoV-2 virus RNA was detected in her husband’s nasopharyngeal swab, who was also convalescent. At that time, she was not vaccinated against COVID-19 (it was early in the pandemic), but her immune system’s response to virus infection, as indicated by her anti-SARS-CoV-2 IgG levels from 3 July, was 1000 U/ml. However, like her husband, she was isolated for 14 days. In August, after a negative RT-PCR test result, she was finally admitted to our institution. She still reported exertional dyspnea and fatigue, but physical exam, laboratory studies, and chest CT scan (we used the same technique as in case 1) did not reveal any significant findings (Figure 2). The anti-SARS-CoV-2-IgG level was 1500 U/ml. Shortly after discharge, her husband was exposed to a person with coronavirus disease and quarantined. In accordance with the epidemic rules in force at that time, the woman was not required to quarantine, but she had to self-isolate until a decision was made by public health inspection.
Discussion
SCENARIO 1:
The SARS-CoV-2 detection by RT-PCR after the patient’s recovery could be a false-positive. There is always a risk of laboratory mistakes, such as testing the wrong sample, contamination by PCR products from previous reactions, and cross-reactivity with other human coronaviruses [9].
SCENARIO 2:
The majority of individuals with dynamic PCR test results are probably no different from those who have prolonged positive results [10]. They have viral shedding at about the limit of detection, so the virus RNA can be detected by RT-PCR test but the results may not be reproducible. The choice of the assay may be of additional importance. NAAT tests detect various regions of the SARS-CoV-2 genome, including ORF, E, N, S RdRp, and others. Targeting at least 2 regions of the viral genome RdRP gene located in the open reading frame ORF1ab and E gene provides higher sensitivity compared to targeting the N gene (our test detected ORF1ab and N genes) [11].
SCENARIO 3:
In the presented cases, the key question is whether we are dealing with breakthrough infection with another SARS-CoV-2 variant due to reduced neutralizing antibody activity in convalescents sera. Doubts arise in the presence of post-COVID-19 symptoms. Observational data of other coronaviruses indicate loss of immunity within 1–3 years after primary infection [12]. Nonetheless, SARS-CoV-2 reinfections are documented [13–15], mostly in young immunocompetent patients and in a few patients with other comorbidities, such as asthma, hypertension, obesity, and diabetes. Tillet et al reported a patient who was infected twice by 2 genetically distinct variants of SARS-CoV-2 [14], but their report does not contain information about the patient’s immune response after the first COVID-19 episode. A case series from Brazil showed that the chance for recurrent COVID-19 is particularly high in patients with reduced antibody response during the initial disease [15]. Therefore, although serology testing is not recommended to diagnose acute SARSCoV-2 infection, in some cases it allows assessment of the risk of subsequent infection. However, the duration of coronavirus antibodies persistence is still unclear and there are doubts that they always offer protective immunity, and the titers of antibodies needed to prevent infection are also unknown unclear. [16]. Currently, there is no SARS-CoV-2 variant of high consequence, but a lineage that escapes from neutralizing antibodies (such as recently described in South Africa) could be a significant problem in the future [17]. At the time of writing, a new variant with many changes in the spike protein has been detected (B.1.1.529, called Omicron). Early studies suggest greater transmissibility and reduced neutralization ability of convalescent sera and vaccinated persons [5]. Accordingly, the newest CDC guidelines removed the recommendation that serological testing can be used to determine the need for quarantine, even in low-risk situations [16]. Therefore, in our cases, detectable antibodies after infection reduced the likelihood of another episode of COVID-19, but only a follow-up period and subsequent hospitalization (with no symptoms of acute infection and no new additional radiographic findings) assured us that quarantine was unnecessary. The example of our patients also shows that diagnostic methods should be adapted to the current epidemiological situation. When diagnostic capacity is insufficient, the ECDC and WHO currently recommend whole SARS-CoV-2 genome sequencing or at least S-gene sequencing, especially among vaccinated individuals or convalescents. When this method is limited, at least diagnostic screening NAAT-based assays should be performed [18]. In our cases, detection of a new SARSCoV-2 variant would indicate a high probability of reinfection.
Conclusions
These 2 cases are from the early pandemic period when any positive RT-PCR result was taken as evidence of infection. The consequence of misdiagnosis was unnecessary isolation of the patients and disruption of the functioning of the healthcare service. At present, it is thought that dynamic RT-PCR results are caused by prolonged persistence of non-infectious viral RNA particles, but the emergence of new SARS-CoV-2 variants requires reassessment of this. Currently, any positive result raises questions about contagiousness and, even in people with recent COVID-19, should be interpreted with caution. Our cases indicate that patients who had dynamic RT-PCR for SARS-CoV-2 require different diagnostics methods from those used in patients with a first-time positive test result. The infectiousness of each patient should be considered individually. Efforts should be made to confirm or exclude reinfection to avoid the consequences of misdiagnosis. Diagnostic methods should also be adapted to the epidemio-logical situation. An example of such an approach is combining diagnostic tests (CT, serological testing, and monitoring clinical symptoms) and identification of SARS-CoV-2 variants to more accurately determine the risk of reinfection.
Figures
References:
1.. , Diagnostic testing for SARS-CoV-2 Interim guidance [Published 11.08.2020. Accessed 13.09.2021]https://www.who.int/publications-detail-redirect/diagnostic-testing-for-sars-cov-2
2.. , Diagnostic testing and screening for SARS-CoV-2 (europa.eu) [Published 21.05.2021. Accessed 13.09.2021]https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing
3.. , COVID-19 – CERTEST biotec IVD diagnostic products [Accessed 13.01.2022]https://www.certest.es/covid-19/
4.. , EUA Authorized Serology Test Performance [Published 12.03.2021. Accessed 13.01.2022]https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
5.. , Epidemiological update: Omicron variant of concern (VOC) – data as of 10 December 2021 [Published 10.12.2021. Accessed 19.12.2021]https://www.ecdc.europa.eu/en/news-events/epidemiological-update-omicron-data-10-december
6.. Bullard J, Durst K, Funk D, Predicting infectious SARS-CoV-2 from diagnostic samples: Clin Infect Dis, 2020; 71(10); 2663-66
7.. Li N, Wang X, Lv T, Prolonged SARS-CoV-2 RNA shedding: Not a rare phenomenon: J Med Virol, 2020; 92(11); 2286-87
8.. , Criteria for releasing COVID-19 patients from isolation [Published 17.06.2020. Accessed 13.09.2021]https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation
9.. Braunstein GD, Schwartz L, Hymel P, Fielding J, False positive results with SARS-CoV-2 RT-PCR tests and how to evaluate a RT-PCR-positive test for the possibility of a false positive result: J Occup Environ Med, 2021; 63(3); 159-62
10.. Gidari A, Nofri M, Saccarelli L, Is recurrence possible in coronavirus disease 2019 (COVID-19)? Case series and systematic review of literature: Eur J Clin Microbiol Infect Dis, 2021; 40(1); 1-12
11.. Corman VM, Landt O, Kaiser M, Detection of 2019 novel coronavirus (2019-nCoV) by real – time RT-PCR: Euro Surveill, 2020; 25(3); 2000045
12.. Sariol A, Perlman S, Lessons for COVID-19 immunity from other coronavirus infections: Immunity, 2020; 53(2); 248-63
13.. Selhorst P, Van Ierssel S, Michiels J: Symptomatic SARS-CoV-2 rein-fection of a health care worker in a Belgian nosocomial outbreak despite primary neutralizing antibody response, 2021; 73(9); e2985-e91
14.. Tillett RL, Sevinsky JR, Hartley PD, Genomic evidence for reinfection with SARS-CoV-2: A case study: Lancet Infect Dis, 2021; 21(1); 52-58
15.. Dos Santos LA, de Gois PG, Silva AMF, Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers and with low Ab titre for SARS-CoV-2: J Infect, 2021; 82(3); 399-406
16.. , Interim Guidelines for COVID-19 Antibody Testing [Published 21.09.2021. Accessed 27.09.2021]https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html
17.. Wibmer CK, Ayres F, Hermanus T, SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma: Nat Med, 2021; 27(4); 622-25
18.. , Methods for the detection and characterisation of SARS-CoV-2 variants – first update 20 Dec 2021 (europa.eu) [Published 20.12.2021. Accessed 13.01.2022]https://www.ecdc.europa.eu/sites/default/files/documents/Methods-for-the-detection-and-characterisationof-SARS-CoV-2-variants-first-update.pdf
Figures
Tables
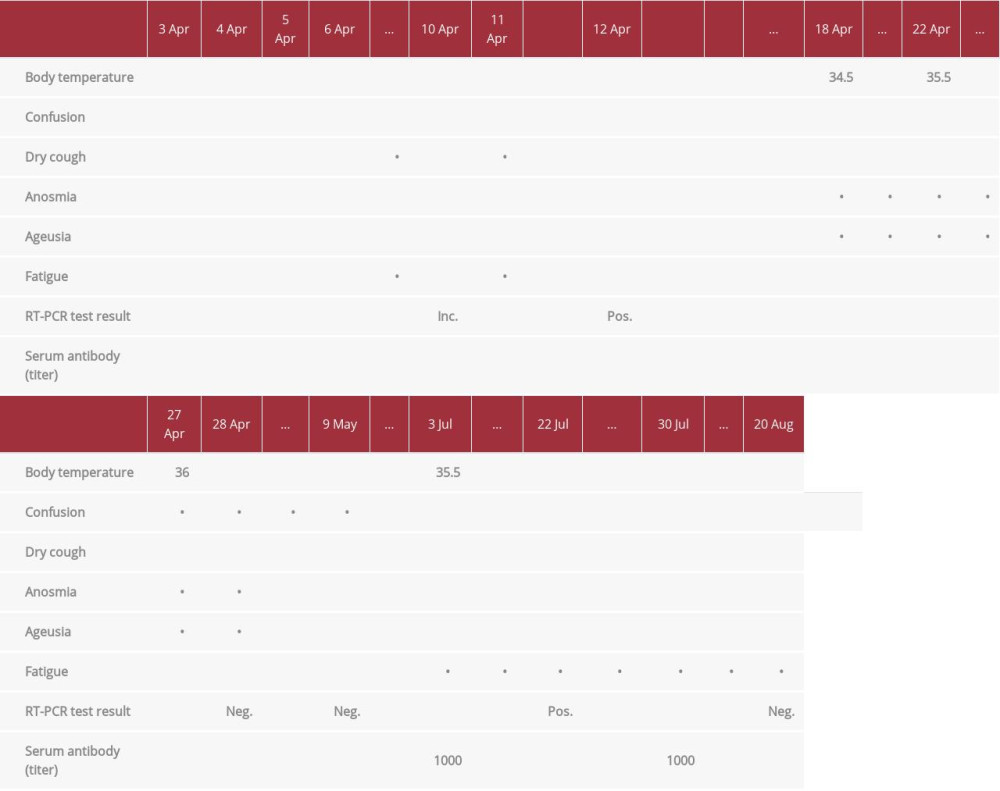 Table 1.. The man’s clinical symptoms in relation to RT-PCR results and anti-SARS-CoV-2 antibodies.
Table 1.. The man’s clinical symptoms in relation to RT-PCR results and anti-SARS-CoV-2 antibodies.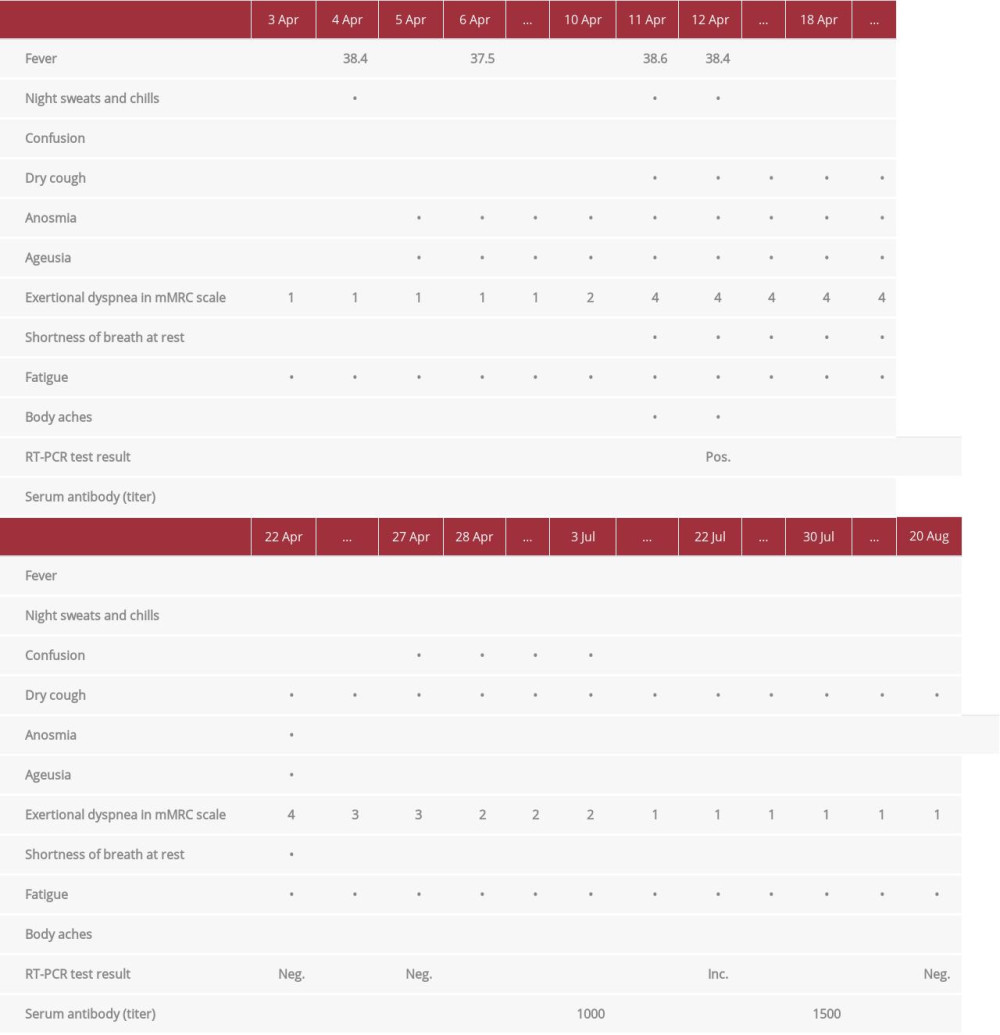 Table 2.. The woman’s clinical symptoms in relation to RT-PCR results and anti-SARS-CoV-2 antibodies.
Table 2.. The woman’s clinical symptoms in relation to RT-PCR results and anti-SARS-CoV-2 antibodies. Table 1.. The man’s clinical symptoms in relation to RT-PCR results and anti-SARS-CoV-2 antibodies.
Table 1.. The man’s clinical symptoms in relation to RT-PCR results and anti-SARS-CoV-2 antibodies. Table 2.. The woman’s clinical symptoms in relation to RT-PCR results and anti-SARS-CoV-2 antibodies.
Table 2.. The woman’s clinical symptoms in relation to RT-PCR results and anti-SARS-CoV-2 antibodies. In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








