12 July 2022: Articles 
A 73-Year-Old Woman Treated for Rheumatoid Arthritis with Lower Rectal Carcinoma Who Underwent Abdominoperineal Resection, Lateral Regional Lymph Node Resection, and Partial Hepatectomy by Hand-Assisted Laparoscopic Surgery (HALS): A Case Report
Unusual clinical course, Unusual or unexpected effect of treatment
Takayuki Tajima1ABCDEFG*, Masaya Mukai2ABCDEFG, Shuji Uda2ABCDE, Hideki Izumi2ABCDE, Daiki Yokoyama2ABCDE, Sayuri Hasegawa2ABCDE, Eiji Nomura2ABCDEDOI: 10.12659/AJCR.936106
Am J Case Rep 2022; 23:e936106
Abstract
BACKGROUND: This report describes the case of a 73-year-old woman treated for rheumatoid arthritis with lower rectal carcinoma who underwent abdominoperineal resection, lateral regional lymph node resection, and partial hepatectomy by hand-assisted laparoscopic surgery (HALS). More recently, HALS has proven to be useful in multiple organ resections.
CASE REPORT: A 73-year-old woman who presented with hematochezia was diagnosed with lower rectal cancer and referred to our hospital. The patient had a history of rheumatoid arthritis and was taking oral nonsteroidal anti-inflammatory drugs. After further evaluation, the patient was diagnosed with stage IV rectal cancer with a metastatic liver lesion and a right lateral lymph node metastasis. All lesions were resected using HALS. A 50-mm longitudinal umbilical incision was created for use as a hand access site, and 3 ports with a diameter of 5 mm each were inserted into the lower abdomen to perform right lateral lymph node dissection and abdominoperineal resection. HALS was performed in the upper abdomen, where the liver was used to partially resect segment S6. The patient was discharged without complications 13 days after the operation.
CONCLUSIONS: In this complex case of advanced rectal carcinoma with liver metastases, use of the HALS surgical method was shown to be possible. Immunomodulatory treatment for rheumatoid arthritis may have influenced the outcome for this patient.
Keywords: Antirheumatic Agents, Arthritis, Rheumatoid, Hand-Assisted Laparoscopy, Hepatectomy, Lymph Node Excision, Rectal Neoplasms, Aged, Carcinoma, Female, Humans, Laparoscopy, Lymph Nodes, Proctectomy
Background
During the past decade or longer, we have reported on more than 600 cases of hand-assisted laparoscopic surgery (HALS) for colorectal cancer with good survival outcomes [1–5]. HALS is used as an alternative to conventional open surgery or laparoscopic surgery; it requires insertion of the hand to maintain the pneumoperitoneum and, consequently, involves direct contact of the surgeon’s arm with the abdominal wound [6]. For surgical treatment of rectal cancer in particular, HALS is now considered an excellent hybrid procedure between conventional laparotomy (CL) and laparoscopic colorectal surgery (LAC), with long-term results comparable to or better than CL in various aspects, including appearance [4,5]. More recently, HALS has also been shown to be useful for multiple organ re-sections, including those involving obstructive colorectal cancer, direct invasion to adjacent organs, and metastases [7–9]. Lastly, we comment on the possibility that immunomodulatory drugs used to treat rheumatoid arthritis may have affected the outcome for this patient [10,11]. This report describes a 73-year-old woman treated for rheumatoid arthritis with lower rectal carcinoma who underwent abdominoperineal resection, regional lymph node resection, and partial hepatectomy by hand-assisted laparoscopic surgery (HALS). More recently, HALS has proven to be useful in multiple organ resections.
Case Report
RECTAL-ANAL RESECTION:
For rectal-anal resection, monitors were placed on the left and right sides of the patient. The surgeon stood on the right side of the patient, and the camera was operated by an assistant on the left side. A 50-mm, longitudinal laparotomy incision was made at the umbilicus, and a visual inspection for intra-abdominal adhesions was performed. A wound re-tractor (Alexis Wound Protector-Retractor S; Applied Medical, Rancho Santa Margarita, CA) was placed into the incision created for use as a hand access site and insufflation was started; at the same time, three 5-mm ports were inserted from the middle of the left and right anterior superior iliac spines and 3 fingertips caudal to the upper pubis (Figure 3). HALS was started with the patient in the right lateral decubitus position with the head down [1–3]. The peritoneal maneuver was performed by the same procedure and technique as standard laparotomy. The fusion fascia of the sigmoid and descending colon were dissected until the layer for total mesorectal excision (TME) was reached (the left ureter and lower abdominal plexus were thereby identified and preserved). Next, the sigmoid mesocolon was incised. The left colonic artery bifurcation was confirmed, ligated, and dissected with a clip at the periphery of the bifurcation. The fascia was dissected at the front of the pouch of Douglas, from the right rectal wall to the caudal side. An incision was made to reach the deep portion of the pelvic floor, taking care not to damage the posterior vaginal wall. The pelvic floor muscles and the anococcygeal ligament were dissected by the HALS procedure. En bloc lymph node dissection was performed with an energy device centered on the right closed ureteral lymph node (no. 283), which appeared metastatic in preoperative imaging. Next, perineal fatty tissue was exposed from the laparotomy incision site, and extralevator abdominoperineal resection was performed colon and the sigmoid plexus were fully resected with complete removal of the external anal sphincter muscle group. Hemostasis was confirmed. The recto-anal resection site was packed and confirmed to be airtight.
PARTIAL HEPATIC RESECTION:
Next, a liver specialist surgeon familiar with HALS joined the surgical team to perform the hepatic resection. The monitors were moved to the right side of the patient, with the surgeon standing at the left side. The camera port was inserted into the upper right abdomen at the location where a drain was likely to be placed external to the liver. At the left side of the epigastrium, two 5-mm ports were inserted to perform the Pringle maneuver at the hepatic hilar (Figure 4A). Preoperative diagnostic imaging and intraoperative ultrasound indicated that a metastatic liver tumor was present at S6 and its safe resection without damaging important vessels was deemed possible. Therefore, a partial hepatic resection was performed. A braid was passed through the foramen of Winslow, and blood circulation was interrupted by the Pringle maneuver by using a Nelaton catheter (No. 9). The bare area of the right lobe of the liver was incised and detached through the left edge of the inferior vena cava, and the liver was mobilized. The planned re-section area was marked on the liver surface after palpation and intraoperative ultrasound examination with a probe previously inserted through the hand access site. Partial hepatectomy was performed with an ultrasonic coagulator (Figure 4B). The main Glisson group was dissected under the clip. After re-section of the metastatic lesion, a drain was placed in an extrahepatic site.
Next, a drain was placed at the bottom of the pelvis using the port insertion site in the lower right abdomen, and the margin of the colon was secured using the HALS procedure to ensure an extra-abdominal route. A single-hole permanent artificial anus was placed in the lower left abdomen, and the operation was completed (Figure 5).
The total operation time was 7 hours 12 minutes, and the volume of blood lost was 701 ml. The duration of surgery was longer than expected because of insertion of a new port before liver resection, preparation for intraoperative ultrasound, and mobilization of the liver from the diaphragm. Exudates from areas of increased exfoliation and the resected rectum constituted 60% of the total volume of blood loss, which was verified by both intraprocedural laparoscopic video and the surgical record.
HISTOPATHOLOGY SPECIMENS:
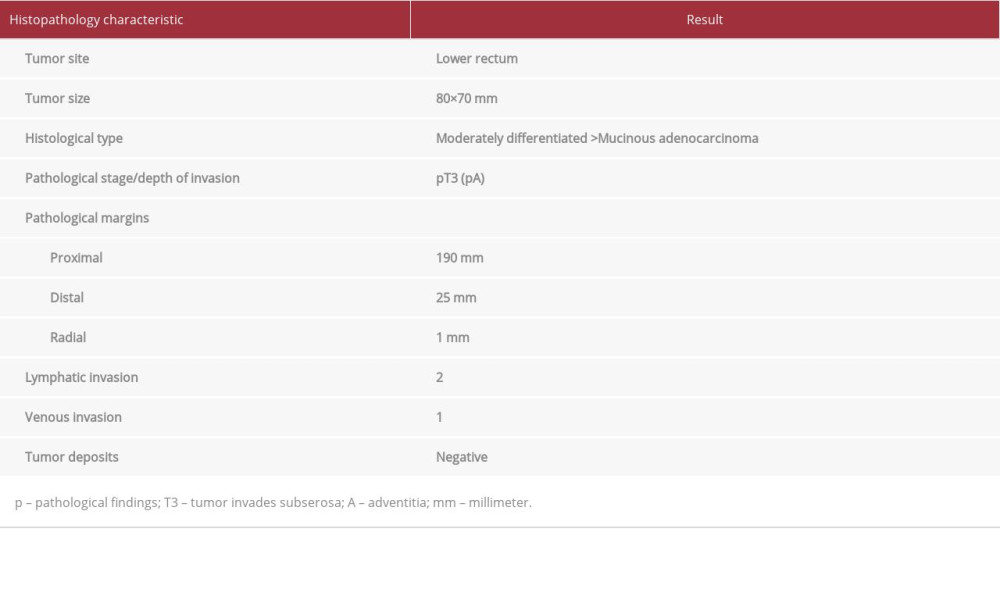
Histopathology results for rectal resection specimens characterized an 80×70 mm, moderately differentiated, stage T3 mucinous adenocarcinoma (Table 1) (Figure 6A). Genetic testing indicated the patient was KRAS-positive, with mutation observed at KRAS codon12 [12]. Of 24 lymph nodes examined, 16 had positive results for metastasis, including the 28-mm right closed foramen lymph node (No. 283D). A 29-mm adenocarcinoma similar to the primary lesion was detected in the resected liver (S6) specimen and diagnosed as a metastatic tumor from the rectum (Figure 6B).
POSTOPERATIVE CLINICAL COURSE:
The patient had an uncomplicated recovery and was discharged from the hospital 13 days after surgery. Before starting adjuvant chemotherapy postoperatively, we performed a CT scan and abdominal ultrasound and confirmed the R0 status. The patient then started XELOX (capecitabine plus oxaliplatin) therapy together with a molecular targeted drug (bevacizumab) at an outpatient clinic. Patients at our institution undergo CT scan and/or abdominal ultrasound every 2 to 3 months after surgery. This routine imaging detected the onset of multiple nodular metastatic foci (<5 mm) in the lungs and liver of this patient 5 months after surgery. During this period, the patient also experienced worsening rheumatoid arthritis, resulting in a change in her rheumatoid arthritis medications (water-soluble prednisolone and iguratimod, an immunomodulating disease-modifying antirheumatic drug). Chemotherapy with tegafur, gimeracil, and oteracil potassium (S-1) plus irinotecan hydrochloride hydrate (IRI) for rectal cancer was initiated. However, the patient died approximately 11 months after the operation.
Discussion
In 2009, we first published a summary of our experiences with HALS in 10 patients with colorectal cancer. Since then, we have published several articles on various aspects of HALS minimally invasive surgery, including the lower costs and good safety and prognosis [5,13–15]. Even in this complex case of advanced rectal carcinoma with liver metastases, the use of the HALS surgical method was shown to be feasible [16].
The use of LAC has become widespread for radical surgery for colorectal cancer in Japan due to the advantages of LAC compared with the CL procedure, which is associated with difficulty visualizing the apex of the prostate and the deep pelvis. With a laparoscope, it is possible to magnify a lesion and share those images, thereby improving safety of the surgical procedure.
HALS initially was used for splenectomy. Because HALS allows the surgeon to use their left hand freely, hemostasis in sudden bleeding can be achieved with fingers, regardless of the size or weight of the tumor. Performing conventional upper abdominal surgery (eg, liver resection) and pelvic surgery (eg, abdominoperineal resection) simultaneously, as in the case described here, requires a large midline incision in the upper and lower abdomen. In contrast, using HALS enables a safe procedure conducted via a small umbilical incision of approximately 5 cm and 5 small port incisions. Moreover, HALS results in decreased wound pain, minimally invasive surgery, and improved ability to perform activities of daily living in the early postoperative period. Additionally, after the HALS operation, a single-hole artificial anus can be constructed safely and easily via retroperitoneal access to prevent parastomal hernia, prostatic prolapse, and internal hernia [17]. Presently, for approximately 7 types of surgical procedures for colorectal cancer, it is possible to perform a total colectomy via 2 to 3 ports [1–3].
When a LAC simulation was performed for this patient, it was judged that the lower abdominal operation could be performed using 5 ports and the upper abdominal operation achieved with 5 ports. Assuming 2 ports could be used for both procedures, a total of 8 or more ports would be required overall. Furthermore, a 50-mm laparotomy was estimated to be needed to obtain the specimen. In comparison, the HALS operation required a 50-mm hand access site at the umbilical site and only five 5-mm ports. The HALS access site also can be used for multiple lesions and other organ resections. Thus, HALS can be considered a safe, reliable procedure that is associated with significantly reduced operative times when performed by experienced surgeons and relatively low-cost procedure for cases such as that reported here [16,18].
HALS is positioned between laparotomy and LACS. We previously reported that a surgeon certified as a specialist surgeon by the Japanese Society of Gastroenterological Surgery can perform the HALS procedure without difficulty [1,19]. A recent article reported that approximately 2% of surgeons who received training in laparoscopy had poor scores in a special perception test. However, because HALS can be performed under laparoscopy using the same procedure as that used for laparotomy, we consider HALS to be a viable procedural option [20].
Recently, final results of a large-scale randomized control trial (JCOG0212) of TME with or without lateral lymph node dissection (LLND) for stage II/III lower rectal cancer did not confirm the noninferiority of TME alone vs TME with LLND [21]. However, other reports have indicated that LLND was associated with significantly decreased local pelvic recurrence without significantly differing survival results, yet a retrospective analysis demonstrated no positive effect of LLND on patient outcome. These conflicting findings indicate that further investigation is warranted [22,23].
Hofheinz et al reported on neoadjuvant chemoradiotherapy (NACRT) for stage II/III lower rectal cancer. NACRT is used prior to resection when preoperative diagnostic imaging indicates nonresectable direct infiltration in the surrounding organs or when complete resection by a single surgery is deemed difficult due to multiple liver metastases in both lobes. However, a 6- to 8-week wait-and-see period often is required after radiotherapy [24]. The appearance or exacerbation of hepatoma and metastatic liver cancer can be assessed during chemotherapy, thereby increasing the likelihood of down-sizing or down-staging in cases of large stage T3/T4 tumors. Moreover, this approach reduces the technical difficulty of surgery and can result in the tumor ablation margin becoming negative (RM0).
In the case presented here, the patient had a hemorrhagic tumor and advanced anemia. Also, the patient had begun experiencing pain and emerging symptoms of stenosis. We presumed this patient had systemic disease with blood-borne metastatic resectable hepatic cancer (H1) [25–28]. Accordingly, we prioritized immediate resection followed by systemic chemotherapy (bevacizumab plus XELOX). In 2016, when we selected a molecularly targeted drug as part of this chemotherapy regimen, clinical guidelines did not indicate superiority of bevacizumab or anti-EGFR (epidermal growth factor receptor) monoclonal antibodies over other drugs. Therefore, we did not consider mutations in RAS and BRAF [27]. Our treatment strategy positioned radiation therapy as an optional treatment in case of postoperative local relapse [29]. However, a recently published Japanese clinical guideline recommends deciding on the chemotherapy regimen after performing molecular testing and determining the tumor mutational burden (TMB) and microsatellite instability (MSI) status [28]. There are no reports of carcinogenicity associated with treatment using iguratimod, an immunomodulatory agent, for postoperative deterioration of rheumatoid arthritis or a comorbid medical disorder. However, some authors have expressed the opinion that long-term caution is indicated regarding the effects of such immunomodulatory therapies on tumor immunity [10,11].
Conclusions
In this complex case of advanced rectal carcinoma with liver metastases, the use of the HALS surgical method was shown to be feasible. Immunomodulatory treatment for rheumatoid arthritis may have influenced the outcome of this patient. A new clinical guideline recommends deciding on the chemo-therapy regimen after performing molecular testing and determining the TMB and MSI status.
Figures
References:
1.. Mukai M, Kishima K, Tajima T, Efficacy of hybrid 2-port hand-assisted laparoscopic surgery (Mukai’s operation) in patients with colorectal cancer: Oncol Rep, 2009; 22; 893-99
2.. Mukai M, [Base techniques of HALS for colorectal cancer. HALS (hand-assisted laparoscopic surgery).], 2014; 19-41, Tokyo [in Japanese], Nankodo Co., Ltd
3.. Mukai M, Yokoyama D, Uda S, [Hand-assisted laparoscopic surgery (HALS) for lower rectal cancer.]: Surgery, 2016; 78; 944-49 [in Japanese]
4.. Tajima T, Mukai M, Yokoyama D, Comparison of hand-assisted surgery (HALS) and conventional laparotomy in patients with colorectal cancer: Final results from single center: Oncol Lett, 2017; 13; 4953-58
5.. Tajima T, Mukai M, Koike T, Better survival after hand-assisted laparoscopic surgery than conventional laparotomy for rectal cancer: Five year results from a single center in Japan: Clin in Surg, 2017; 2; 1368
6.. Loungnarath R, Fleshman JW, Hand-assisted laparoscopic colectomy techniques: Semin Laparosc Surg, 2003; 10(4); 219-30
7.. Mukai M, Kishima K, Iizuka S, Curative resection by hybrid 2-port HALS in a patient with advanced cecal cancer invading the urinary bladder: A case report: Oncol Rep, 2009; 21; 1385-89
8.. Mukai M, Yokoyama D, Tajima T, [Primary versus staged resection for obstructing colorectal cancer.]: Journal of Clinical Surgery, 2018; 73; 32-38 [in Japanese]
9.. Mukai M, Yokoyama D, Hasegawa S, [Three-port HALS for double colorectal cancer.]: Operation, 2018; 72; 1293-98 [in Japanese]
10.. Strangfeld A, Zing A, Assessing cancer risk of cytokine inhibitors in RA: Nat Rev Rheumatol, 2010; 6; 126-27
11.. Raaschou P, Söderling J, Turesson C, Askling J, ARTIS Study Group: Tumor necrosis factor inhibitors and cancer recurrence in Swedish patients with rheumatoid arthritis: A nationwide population-based cohort study: Ann Intern Med, 2018; 169(5); 291-99
12.. : Japanese Classification of Colorectal, appendiceal, and Anal Carcinoma, 2018; P7-P27, Tokyo, Japan, Kanehara Shuppan Co. Ltd.
13.. Tajima T, Mukai M, Yamazaki M, Comparison of hand-assisted laparoscopic surgery and conventional laparotomy for colorectal cancer: Interim results from a single institution: Oncol Lett, 2014; 8; 627-32
14.. Tajima T, Mukai M, Noguchi W, Comparison of hand-assisted laparoscopic surgery and conventional laparotomy for rectal cancer: Interim results from a single center: Mol Clin Oncol, 2015; 3; 533-38
15.. Tajima T, Mukai M, Yokoyama D, Comparison of hand-assisted laparoscopic surgery (HALS) and conventional laparotomy in patients with colorectal cancer: Final results from a single center: Oncol Lett, 2017; 13; 4953-58
16.. Meshikhes AW, Controversy of hand-assisted laparoscopic colorectal surgery: World J Gastroenterol, 2010; 16(45); 5662-68
17.. Londone-Schmmer EE, Leong APK, Philips RKS, Life table analysis of stomal complications following colostomy: Dis Colon Rectum, 1994; 37; 916-20
18.. Cima RR, Pendlimari R, Houlbar SD, Utility and short-term outcomes of hand-assisted laparoscopic colorectal surgery: A single-institution experience in 1103 patients: Dis Colon Rectum, 2011; 54; 1076-81
19.. Mukai M, Sekido Y, Hoshikawa T, Two-stage treatment (Mukai’s method) with hybrid 2-port HALS (Mukai’s operation) for complete bowel obstruction by left colon cancer or rectal cancer: Oncol Rep, 2010; 24; 25-30
20.. Henn P, Gallagher AG, Nugent E, Visual spatial ability for surgical trainees: Implications for learning endoscopic, laparoscopic surgery and other image-guided procedures: Surg. Endosc, 2018; 32; 3634-39
21.. Fujita S, Mizusawa J, Kanemitsu Y, Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): A multicenter, randomized controlled, noninferiority trial: Ann Surg, 2017; 266; 201-7
22.. Ozawa H, Kotake K, Hosaka M, Impact of lateral pelvic lymph node dissection on the survival of patients with T3 and T4 lower rectal cancer: World J Surg, 2016; 40; 1492-99
23.. Kinugasa T, Akagi Y, Shirouz K, Benefit of lateral lymph node dissection for rectal cancer: Long-term analysis of 944 cases undergoing surgery at a single center (1975–2004): Anticancer Res, 2014; 34; 4633-39
24.. Hofheinz RD, Wenz F, Post S, Matzdorff A, Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: A randomized, multicenter, non-inferiority, phase 3 trial: Lancet Oncol, 2012; 13; 579-88
25.. Tamura H, Shimada Y, Yagi Y, [Three cases of Stage lV low rectal cancer with lateral pelvic lymph node metastasis.]: Gan To Kagaku Ryoho, 2015; 42; 2303-5 [in Japanese]
26.. Hadden WJ, de Reuver PR, Brown K, Resection of colorectal liver metastasies and extrahepatic disease: A systematic review and proportional meta-analysis of survival outcomes: HPB (Oxford), 2016; 18; 209-20
27.. : JSCCR Guidelines 2016 for the Treatment of Colorectal Cancer, 2016; 18-38, Tokyo, Kanehara & Co., Ltd
28.. : JSCCR Guidelines 2022 for the Treatment of Colorectal Cancer, 2022; 31-42, Tokyo, Kanehara & Co., Ltd.
29.. Yokoyama D, Mukai M, Uda S, Efficacy of modified bevacizumab-XELOX therapy in Japanese patients with stage lV recurrent or non-resectable colorectal cancer: J Gastrointest Oncol, 2021; 12; 527-34
Figures
In Press
04 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.941835
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943042
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250