23 August 2022: Articles 
Thrombotic Microangiopathy Secondary to Disseminated Varicella Zoster Virus Infection in an Adult Patient
Unusual clinical course, Challenging differential diagnosis
Neama Luqman1ABCDEFG*, Rama Bakie1ABCDEFG, Waqar H. GabaDOI: 10.12659/AJCR.936294
Am J Case Rep 2022; 23:e936294
Abstract
BACKGROUND: Thrombotic microangiopathy (TMA) is a life-threatening condition caused by small-vessel platelet microthrombi. While various disease triggering factors, including infections, have been well described, there have been few reports of an association between TMA and varicella zoster virus (VZV) infection. VZV infection is rare among people age 20 and older, and infection-induced TMA is mostly reported in the pediatric age group. We report a case of TMA induced by a disseminated VZV infection in an adult.
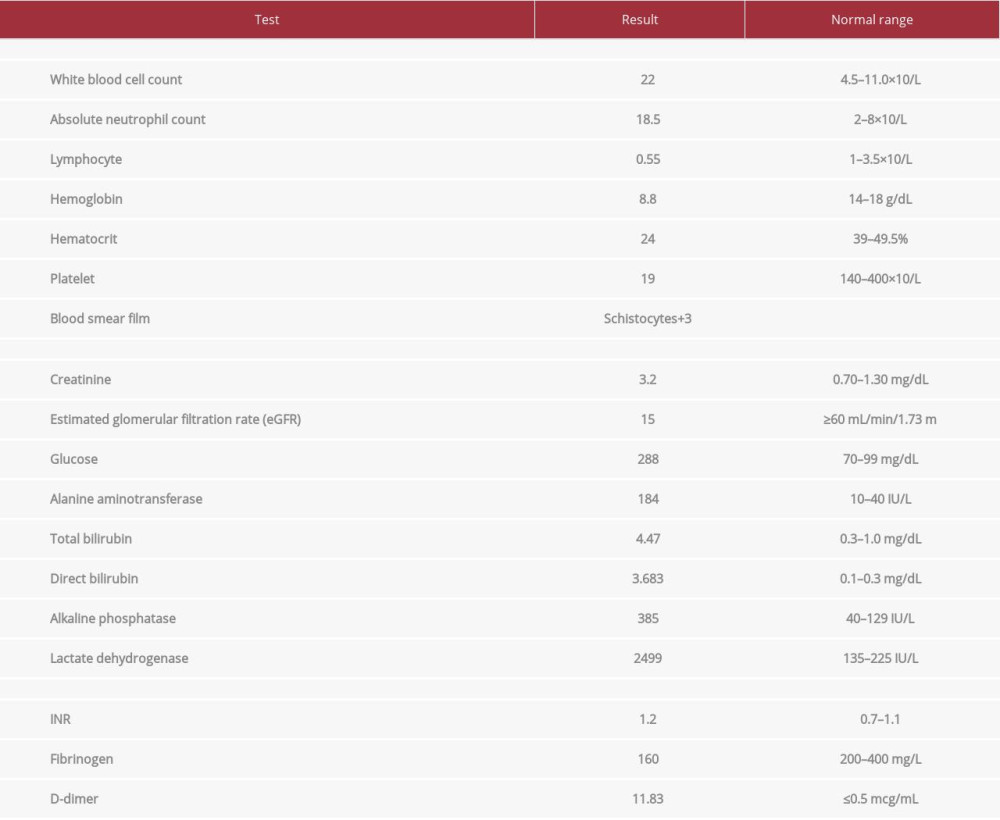
CASE REPORT: A 43-year-old man presented with a 3-day duration of fever, headache, vomiting, and bloody diarrhea. He also reported body rash after a recent contact with a few roommates with chickenpox. On presentation, the patient developed convulsive seizures. His laboratory test results were significant for acute kidney injury (AKI) and thrombocytopenia. Atypical hemolytic uremic syndrome (aHUS) and thrombotic thrombocytopenic purpura (TTP) were suspected but further diagnostic testing was negative. The treatment plan included acyclovir, therapeutic plasma exchange, and high-dose oral prednisolone (1.5 mg/kg). The clinical and biochemical profile significantly improved, and the patient was discharged home.
CONCLUSIONS: TMA is a life-threatening hematological emergency with a high mortality rate. Compared to the pediatric population, VZV infection tends to be more severe in the adult age group. This case demonstrates that a high index of suspicion for TMA in adult patients with VZV who present with thrombocytopenia, even when there is no definitive diagnosis, can result in early management with favorable outcome.
Keywords: Atypical Hemolytic Uremic Syndrome, Hemolytic-Uremic Syndrome, Purpura, Thrombotic Thrombocytopenic, Thrombotic thrombocytopenic purpura, acquired, Varicella Zoster Virus Infection, Thrombotic Microangiopathies, Adult, Chickenpox, Child, Herpes Zoster, Herpesvirus 3, Human, Humans, Male, young adult
Background
TMA is a syndrome characterized by microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and renal failure. It encompasses the spectrum of classical thrombotic thrombocytopenic purpura (TTP) described first by Moschowitz [1], and HUS described 3 decades later by Gasser et al [2]. TMA has been associated with various triggering factors, including infections, illicit drug use, neoplasms, connective tissue disorders, and pregnancy [3–5]. Direct endothelial injury is the proposed pathogenesis mechanism for the development of TMA. It is characterized by thickening of the arterioles, endothelial swelling, intraluminal platelet thrombi, and fragmentation of red blood cells (RBC) as blood flows across the partially occluded microcirculation. A few reports, such as Basnayake (2020) [6], showed an association between TMA and VZV infection, and the majority were in the pediatric age group. In the present report, we discuss a case of TMA induced by a disseminated VZV infection in an adult with a diagnostic challenge but a successful outcome.
Case Report
OUTCOME AND FOLLOW-UP:
Upon discharge, the patient had improved, and his renal functions, platelet count, and LDH levels had normalized (Figure 2). After a 21-day stay in the hospital, the patient was released on a tapering dose of steroids with a follow-up arranged in 2 weeks. Unfortunately, the patient traveled to his home country and was lost further follow-up.
Discussion
Viruses are important in the development of TMA; many are only linked to HUS or aHUS, but some can cause TTP. The pathophysiology of TMA induced by viruses is unknown. On the other hand, direct endothelial cell damage appears to be an important pathology trigger [7–9]. Furthermore, host genetic or environmental susceptibility factors may provide a favorable environment for viruses to initiate the chain of events that result in TMA’s clinical manifestations. Although certain reports of viral-related TMA are based on anecdotal evidence, such correlations cannot be ruled out [7–10].
Varicella is predominantly a childhood disease characterized by vesicular exanthema frequently accompanied by fever and malaise. Human VZV disseminates to the viscera during viremia and multiplies in reticuloendothelial tissues. Varicella usually manifests as mild to moderate illness, but serious complications (eg, meningoencephalitis, meningitis, vasculitis affecting small or large vessels, pneumonia, and hemorrhages) can arise. There is only 1 previous report of this type of HUS following varicella infection [11].
Primary VZV infection in adults has a more severe presentation, including interstitial pneumonia, in comparison to younger age groups. In addition, the infection produces severe, disseminated diseases in immunocompromised individuals. More than 90% of people are infected before adolescence, with 13–16 cases per 1000 people per year. In tropical climates, however, VZV infection occurs later in life. Varicella has a peak incidence in the late winter and spring, and epidemics tend to occur every 2–5 years [12,13].
Fever and a self-limiting rash on the skin, and occasionally the mucosa, are symptoms of varicella. Headaches, malaise, and a loss of appetite are also common. Macules are the first signs of the rash and rapidly progress to papules, followed by a vesicular stage and crusting of lesions. VZV is highly infectious, and transmission occurs by direct contact with skin lesions or respiratory aerosols from infected individuals. Central nervous system complications include self-limiting cerebellar ataxia in 1 in 4000 cases, meningitis, meningoencephalitis, and vasculopathy. Stroke may occur months after varicella, secondary to VZV vasculopathy, and are not always easy to diagnose [12,13].
Our patient presented with a varicella infection associated with macular rash and fever followed by renal failure and seizure.
He was investigated for TTP, but ADAMTS13 testing was reported to be normal. Given the presence of MAHA, thrombocytopenia, and renal failure, we believe that this was a case of TMA, with a high probability of an atypical form of HUS. The patient showed improvement after receiving multiple sessions of plasma exchange and steroids.
New algorithms for managing MAHA, thrombocytopenia, and renal failure have developed because the frequency of different kinds of HUS varies by age [12,13]. In our case, the negative E. coli 0157: H7 shiga-like exotoxin and the shiga toxin assays excluded atypical HUS, and TTP was excluded by the lack of severe ADAMTS13 deficiency, resulting in lack of a definitive diagnosis. The present consensus is that plasma exchange for 5 days should be used as a first-line treatment [12]. If an inadequate response to medication fails to normalize LDH, platelet count, or lower creatinine level by more than 25%, eculizumab therapy should be started, but our patient’s LDH and creatinine levels improved despite the lag in platelet count improvement.
Conclusions
TMA is a life-threatening hematological emergency with a high mortality rate if not identified and treated early. Identifying the etiology may help guide the management.
VZV infection tends to be more severe in the adult age group compared to the pediatric population. Many viral infections are known to be associated with TMA [4,7].
To the best of our knowledge, this is the first reported case of TMA as a complication of VZV infection in an adult. A high index of suspicion for TMA in adult patients with VZV who present with thrombocytopenia, even when there is no definitive diagnosis, can result in early management with favorable outcome.
Figures
References:
1.. Moschcowitz E, An acute febrile pleiochromic anemia with hyaline thrombosis of the terminal arterioles and capillaries; an undescribed disease: Am J Med, 1952; 13(5); 567-69
2.. Gasser C, Gautier E, Steck A, [Hemolytic-uremic syndrome: Bilateral necrosis of the renal cortex in acute acquired hemolytic anemia]: Schweiz Med Wochenschr, 1955; 85(38–39); 905-9 [in German]
3.. Remuzzi G, HUS and TTP: Variable expression of a single entity: Kidney Int, 1987; 32(2); 292-308
4.. Ruggenenti P, Remuzzi G, Pathophysiology and management of thrombotic microangiopathies: J Nephrol, 1998; 11(6); 300-10
5.. Booth KK, Terrell DR, Vesely SK, George JN, Systemic infections mimicking thrombotic thrombocytopenic purpura: Am J Hematol, 2011; 86(9); 743-51
6.. Basnayake BMDB, Wazil AWM, Nanayakkara N, Atypical hemolytic uremic syndrome: A case report: J Med Case Rep, 2020; 14(1); 11
7.. Lopes da Silva R, Viral-associated thrombotic microangiopathies: Hematol Oncol Stem Cell Ther, 2011; 4(2); 51-59
8.. Java A, Edwards A, Rossi A, Cytomegalovirus-induced thrombotic microangiopathy after renal transplant successfully treated with eculizumab: Case report and review of the literature: Transpl Int, 2015; 28(9); 1121-25
9.. Johnson S, Waters A, Is complement a culprit in infection-induced forms of haemolytic uraemic syndrome?: Immunobiology, 2012; 217(2); 235-43
10.. Coppo P, Adrie C, Azoulay E, Infectious diseases as a trigger in thrombotic microangiopathies in intensive care unit (ICU) patients?: Intensive Care Med, 2003; 29(4); 564-69
11.. Kwon T, Belot A, Ranchin B, Varicella as a trigger of atypical haemolytic uraemic syndrome associated with complement dysfunction: Two cases: Nephrol Dial Transplant, 2009; 24(9); 2752-54
12.. Akesson A, Zetterberg E, Klintman J, At the cross section of thrombotic microangiopathy and atypical hemolytic uremic syndrome: A narrative review of differential diagnostics and a problematization of nomenclature: Ther Apher Dial, 2017; 21(4); 304-19
13.. Mueller NH, Gilden DH, Cohrs RJ, Varicella zoster virus infection: Clinical features, molecular pathogenesis of disease, and latency: Neurol Clin, 2008; 26(3); 675-viii
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250