10 October 2022: Articles 
A -Infected Patient Who Could Not Tolerate Oral Intake Successfully Treated Using an Intravenous Tedizolid-Containing Regimen
Unusual or unexpected effect of treatment
Haruka Karaushi12ABCD, Masafumi Seki1ABCDEFG*, Yutaka MiyawakiDOI: 10.12659/AJCR.937485
Am J Case Rep 2022; 23:e937485
Abstract
BACKGROUND: Mycobacterium tuberculosis (M. tuberculosis) is usually treated by oral antimycobacterial agents, including rifampicin, ethambutol, and pyrazinamide, but the treatment regimen with intravenous and/or intramuscular antimycobacterial agents for patients who cannot take medications orally remains unclear.
CASE REPORT: A 77-year-old man with chronic renal failure had an esophageal-skin fistula after he had surgeries for removal of esophageal and gastric cancers and reconstruction using jejunum, and he showed a cavity, tree-in-bud formation, and pleural effusions in his left upper lung fields on his chest X-ray after treatment of cellulitis and bacteremia/candidemia by meropenem, teicoplanin, and micafungin. M. tuberculosis was isolated from his sputum and exudate fluid from the reconstructed esophageal-skin fistula. Although he could not take antimycobacterial agents orally, treatment was started with intravenous agents combining levofloxacin (LVFX) every other day, isoniazid (INH), and linezolid (LZD). However, his platelets were decreased 21 days after treatment started, and it was thought to be an adverse effect of LZD and/or INH. After changing LZD to tedizolid (TZD), in addition to changing from INH to intramuscular streptomycin twice per week, his platelet counts increased. Intravenous TZD could be continued, and it maintained his condition without exacerbations of thrombocytopenia and renal failure. The M. tuberculosis disappeared, and the abnormal chest X-ray shadows were improved 2 months after the start of treatment.
CONCLUSIONS: Administration of intravenous TZD, in addition to intravenous LVFX and intramuscular SM in combination, might be a candidate regimen for M. tuberculosis patients who cannot take oral medications.
Keywords: Renal Insufficiency, Chronic, Immunocompromised Host, Anti-Bacterial Agents, Aged, Antitubercular Agents, Cutaneous Fistula, Ethambutol, Humans, Isoniazid, Levofloxacin, linezolid, Male, meropenem, micafungin, Mycobacterium tuberculosis, Oxazolidinones, Pyrazinamide, Rifampin, Streptomycin, Teicoplanin, Tetrazoles, Tuberculosis
Background
Tuberculosis remains the leading cause of death from an infectious disease in adults worldwide, with more than 10 million people becoming newly sick from tuberculosis each year. Although little has changed in the treatment of drug-susceptible tuberculosis, data on increased efficacy with new and re-purposed drugs led the World Health Organization (WHO) to recommend all-oral therapy for drug-resistant tuberculosis for the first time ever in 2018 [1].
However, some tuberculosis patients, such as elderly persons, aspiration pneumonia cases, and postoperative patients, cannot be treated with oral antimycobacterial drugs; therefore, intravenous and/or intramuscular administration of the drugs must be selected for the treatment of their tuberculosis, although
Recently,
This case and the related study were approved by the Institutional Review Board of Saitama Medical University International Center on May 27, 2022 and registered as UMIN000047689.
Case Report
A 77-year-old man with chronic kidney disease (CKD) underwent surgeries in our hospital for removal of esophageal and gastric cancers 2 years earlier along with reconstruction of the esophagus by jejunum several times, but he had developed an esophageal-skin fistula. He could not take food orally and had been started on intravenous hyperalimentation (IVH). In 2019, he developed a high fever and an erosion around the esophageal-skin fistula and was admitted to our hospital again. Laboratory data on admission were as follows: white blood cell (WBC) count, 14.5×103/µL, with 94.0% neutrophils, 3.8% lymphocytes, 2.0% monocytes, 0.1% eosinophils, and 0.1% basophils; platelet count, 224×103/µL; hemoglobin, 10.6 g/dL; blood urea nitrogen, 114.2 g/L; serum creatinine, 3.98 mg/dL; aspartate aminotransferase (AST), 15 U/L; alanine aminotransferase (ALT), 8 U/L; and C-reactive protein, 10.794 mg/dL. Cellulitis was suspected, and he was started on cefazolin 1 g/day followed by meropenem 1 g/day. Methicillin-resistant
On day 12, the fever was decreased, but dyspnea appeared, and his chest X-ray and computed tomography (CT) showed a cavity, tree-in-bud appearance, infiltration shadows, and pleural effusions (Figure 1). No bacteria and fungi were detected, but acid-fast bacilli were detected from his sputum and slight pus from the esophageal-skin fistula.
Because the patient appeared to be severely ill and could not tolerate oral intake, the first-line regimen, which includes oral RIP, EB, and PZA, could not be given; therefore, combination antibiotic therapy with intravenous INH 200 mg/day, LVFX 250 mg every other day, and LZD 1200 mg/day was started, because it is known to have anti-mycobacterial effects, is immunomodulatory, and dose adjustment is not necessary even in CKD patients (Figure 2).
His condition subsequently improved, and blood tests showed that WBC decreased to 3110/µL, and C-reactive protein returned to 6.001 mg/dL. However, platelet counts decreased, which is an adverse effect of LZD, from 224×103/µL to 23×103/µL more than 2 weeks later. Therefore, LZD was changed to intravenous TZD, an oxazolidinone similar to LZD but not known to cause thrombocytopenia [5,6]. In addition, intravenous administration of INH was also suggested as the cause of thrombocytopenia [7], and it was changed to intramuscular administration of SM 300 mg twice/week. The patient’s condition remained stable; his platelet count increased to 167×103/µL, and serum C-reactive protein decreased to 1.41 mg/dL.
Discussion
There is a need for newer compounds and regimens that have a sterilizing effect not only in patients with drug-resistant
In the present case, an
SM, an aminoglycoside, has good activity against mycobacteria, with susceptibilities usually greater than 90% [8,9]. Intramuscular administration of SM was finally used in this case, and it might be considered an alternative candidate drug for
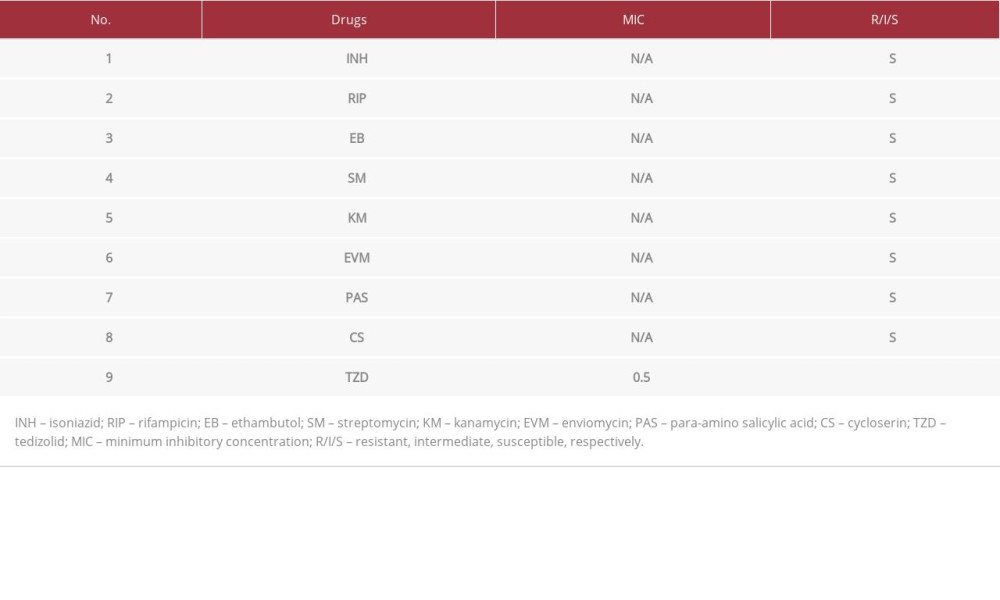
In addition, fluoroquinolones have been available most widely, but there is evidence that resistance to drugs of this class increases after repeated use [9]. In the present case, LVFX was used because the causative organism was found to be susceptible to LVFX later (Table 1), and it was one of the few agents that could be used intravenously. Moxifloxacin (MFLX) might be a good alterative candidate, but the intravenous formulation of MFLX is not available in Japan.
In the present case, LZD, which is an oxazolidinone antibiotic to which the organism showed good susceptibility
Furthermore, TZD has become available as an oxazolidinone with immunomodulatory effects similar to LZD, but showing more effectiveness at half the dose of LZD [18,19]. The sterilizing effect of intermittent TZD for pulmonary tuberculosis was shown, and a TZD dose of 200 mg/day or 700 mg twice a week is recommended for testing in patients; the intermittent TZD dosing schedule is suggested to be much safer than daily LZD [4].
Conclusions
We present the case of a CKD patient with pulmonary
Figures
References:
1.. Furin J, Cox H, Pai M, Tuberculosis: Lancet, 2019; 393; 1642-56
2.. Nahid P, Mase SR, Migliori GB, Treatment of drug-resistant tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline: Am J Respir Crit Care Med, 2019; 200; e93-142
3.. Ruiz P, Causse M, Vaquero M, Casal M: Antimicrob Agents Chemother, 2019; 63; e01939-18
4.. Srivastava S, Deshpande D, Nuermberger E, The sterilizing effect of intermittent tedizolid for pulmonary tuberculosis: Clin Infect Dis, 2018; 67; S336-41
5.. Mensa Vendrell M, Tasias Pitarch M, Salavert Lletí M, Safety and tolerability of more than six days of tedizolid treatment: Antimicrob Agents Chemother, 2020; 64; e00356-20
6.. Seki M, Kamioka Y, Takano K: Am J Case Rep, 2020; 21; e924642
7.. Kuwabara G, Tazoe K, Imoto W, Isoniazid-induced immune thrombocytopenia: Intern Med, 2021; 60; 3639-43
8.. Chen J, Zhao L, Mao Y: Front Microbiol, 2019; 10; 1977
9.. Shen Y, Wang X, Jin J: Biomed Res Int, 2018; 2018; 4902921
10.. Song Y, Wu J, Yan H, Chen J, Peritoneal dialysis-associated nontuberculous mycobacterium peritonitis: A systematic review of reported cases: Nephrol Dial Transplant, 2012; 27; 1639-44
11.. Conradie F, Diacon AH, Ngubane N, Treatment of highly drug-resistant pulmonary tuberculosis: N Engl J Med, 2020; 382; 893-902
12.. Wasserman S, Meintjes G, Maartens G, linezolid in the treatment of drug-resistant tuberculosis: The challenge of its narrow therapeutic index: Expert Rev Anti Infect Ther, 2016; 14; 901-15
13.. Bhan U, Podsiad AB, Kovach MA, Linezolid has unique immunomodulatory effects in post-influenza community acquired MRSA pneumonia: PLoS One, 2015; 10; e0114574
14.. Kakeya H, Seki M, Izumikawa K, Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: A multicenter, open-label, randomized study: PLoS One, 2014; 14; e91293
15.. Seki M, Sakata T, Toyokawa M: Intern Med, 2016; 55; 307-10
16.. Bogard KN, Peterson NT, Plumb TJ, Antibiotic dosing during sustained low-efficiency dialysis: Special considerations in adult critically ill patients: Crit Care Med, 2011; 39; 56-70
17.. El-Naggari M, El Nour I, Al-Nabhani D: J Infect Public Health, 2016; 9; 192-97
18.. Housman ST, Pope JS, Russomanno J, Pulmonary disposition of tedizolid following administration of once-daily oral 200-milligram tedizolid phosphate in healthy adult volunteers: Antimicrob Agents Chemother, 2012; 56; 2627-34
19.. Lemaire S, Van Bambeke F, Appelbaum PC, Tulkens PM, Cellular pharmacokinetics and intracellular activity of torezolid (TR-700): Studies with human macrophage (THP-1) and endothelial (HUVEC) cell lines: J Antimicrob Chemother, 2009; 64; 1035-43
20.. Yuste JR, Serrano-Alonso M, Carmona-Torre F, Efficacy and safety of long-term use of tedizolid after liver transplantation in an adolescent with pulmonary tuberculosis: J Antimicrob Chemother, 2019; 74; 2817-19
Figures
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250