22 December 2022: Articles 
Severe COVID-19 in 2 Kidney Transplant Patients in Gabon
Management of emergency care, Rare coexistence of disease or pathology
Guignali Laurette Mangouka1ABCDEFG, Berthe Amélie Iroungou2ABCDEF*, Pamela Moussavou-Boundzanga3EF, Jean Raymond Nzenze1GDOI: 10.12659/AJCR.938003
Am J Case Rep 2022; 23:e938003
Abstract
BACKGROUND: Kidney failure is a public health problem that may require transplantation for patient survival and for those at risk of developing infectious diseases such as COVID-19 due to severe immunosuppression. We report the case of 2 kidney transplant patients who contracted COVID-19.
CASE REPORT: Patient 1: A 60-year-old Gabonese man presented with 8 days of wet cough, fever, and myalgias associated secondarily with dyspnea, without anosmia or ageusia. His medical history included renal transplant for malignant nephro-angiosclerosis and high blood pressure. The oxygen saturation level subsequently fell to 89-90%. The diagnosis of acute hypoxic respiratory failure secondary to COVID-19 pneumonia with heart and acute renal failure on renal transplant was made based on clinical symptoms, lung imaging results, and a positive SARS-CoV-2 nasal swab PCR test. Patient 2: A 79-year-old Gabonese man presented with 10 days of dry cough associated with intermittent fevers not quantified, anorexia, and fatigue. The patient’s medical history was high blood pressure, diabetes mellitus, and renal transplantation. Oxygen saturation level decreased to 85-89% in ambient air. Clinical signs and chest CT scan showed 70% lung lesions with large areas of ground-glass opacity with essentially peripheral distribution of both lungs associated with crazy paving, condensation, bronchiectasis, and arterial dilatation, suggesting severe COVID-19.
CONCLUSIONS: Those 2 presentations highlight the fact that a severe clinical form of COVID-19 associated with acute renal failure and kidney transplant can be fatal. Kidney transplantation is a risk factor for poor prognosis in patients with severe COVID-19 and greatly worsens the mortality rate of immunocompromised patients.
Keywords: Adult Multisystem Inflammatory Disease, COVID-19 Related, respiratory distress syndrome, Acute Kidney Injury, Kidney Transplantation, Gabon, Male, Humans, Middle Aged, Aged, COVID-19, SARS-CoV-2, Cough, Hypertension
Background
Since December 2019, the world has been invaded by a new virus named SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2), which has caused many deaths [1]. However, the African continent has been relatively spared from severe forms of Coronavirus disease 2019 (COVID-19). In Gabon, a study carried out in 2021 reported that 3.7% of patients with COVID-19 had severe forms, 33.7% had moderate forms, and 62.6% had simple forms [2]. Therefore, in this region of the world, with the exception of South Africa, few descriptions are currently available in the medical literature of the impact of severe forms of COVID-19 in kidney transplant recipients. Conversely, several case series and individual cases have been reported from other countries, mainly China, USA, Italy, and Spain, highlighting the fact that chronic immunosuppression after kidney transplantation places patients at risk of COVID-19 [3]. In 2020, epidemiological studies conducted in Europe and the USA on COVID-19 in kidney transplant patients showed that the average incidence rate of infection ranged from 14/1000 to 17.7/1000. This could suggest that the observed incidence of COVID-19 during these periods would be 2 to 5 times higher in kidney transplant recipients than in the general population [4]. In addition, the management of kidney transplant patients is a therapeutic challenge in countries without transplant centers [5,6].
Thus, for medical practice in tropical areas, particular attention should be paid to clinical forms of COVID-19 in kidney transplant patients, because this target population might be at risk of developing a severe form of COVID-19. Herein, we report the cases of 2 Gabonese kidney transplant recipients who developed COVID-19 during the second and fourth waves of the disease in Gabon.
Case Reports
PATIENT 1:
On December 2, 2019, a 60-year-old Gabonese man presenting with 8 days of wet cough, fever, and myalgia associated secondarily with dyspnea, without anosmia or ageusia, was admitted to the COVID service for diagnostic and therapeutic management.
The patient’s medical history included right then left renal transplant for malignant nephro-angiosclerosis in 1991 and 2009, high blood pressure, hepatitis C virus treated and cured in 2006, and an arteriovenous fistula on his left arm. His usual treatments included antihypertensive and immunosuppressive drugs (cyclosporine and mycophenolate mofetil).
When the clinical signs appeared, the patient self-medicated with an antibiotic (amoxicillin clavulanic acid) and an antimalarial treatment. He reported no urinary symptoms and no digestive signs such as nausea, vomiting, or abdominal pain. He had not been vaccinated against SARS-CoV-2. A few days later, his health worsened with the onset of stage II/III dyspnea and a drop in oxygen level to 96%, leading to hospitalization in the Internal Medicine Department (COVID Unit) of the Omar Bongo Ondimba Army Training Hospital, Libreville, Gabon.
On admission to the COVID Unit, his weight was 67 kg, with body mass index 20.6, temperature 37.5°C, blood pressure 150/70 mmHg, pulse rate 63 beats per minute, respiratory rate 22 breaths per minute, and oxygen saturation level 93%, but this subsequently fell to 89–90%. Physical examination showed mild edema of the lower limbs, spontaneous turgidity of the jugulars, audible heart sounds with systolic and aortic murmurs of intensity 3/6, and no murmurs in the major vascular axes. He was well oriented in time and space, with a Glasgow coma scale score of 15/15, and no sensory or motor deficits. He had diffused and bilateral crackling rales.
The blood tests results were: white blood cells 3300/mm3, neutrophils 2050/mm3, lymphocytes 600/mm3, monocytes 650/mm3, eosinophils 1/mm3, basophils 1/mm3, hemoglobin (Hb) 8.4 g/dl, mean corpuscular volume (MCV) 81.2 fl, platelets 112 000/mm3 (Table 1) C-reactive protein (CRP) 90 mg/l, D-dimers 1300 µg/l, natremia 133.2 mmol/L, kalemia 3.66 mmol/L, lactic-dehydrogenase (LDH) 177I U/l, blood glucose 14.71 mmol/l, aspartate aminotransferase (AST) 98 UI/l, alanine aminotransferase (ALT) 66 UI/l, total bilirubin 13 µmol/l, direct bilirubin 8 µmol/l, Urea 16.27 mmol/l, creatinine 326 µmol/l, and creatinine clearance 20 ml/min (Table 2). A malaria test was negative. The SARS-CoV-2 polymerase chain reaction (PCR) nasal swab test was positive and he was negative for the SARS-CoV-2 antigen test.
An electrocardiogram showed regular heartbeat and no signs of arrhythmia or repolarization. Doppler echocardiography showed dilated left ventricle, preserved left ventricular ejection fraction, homogeneous kinetics, and bi-atrial dilatation, with minimal aortic and mitral insufficiency. The right ventricle was not dilated, and he was normokinetic, with dry pericardium. The inferior vena cava was dilated.
A chest CT scan without injection of iodinated contrast medium revealed a severe form of COVID-19 with extensive lung lesions of 50% to 55% (Figure 1). An axial slice in the parenchymal window showed left basal subpleural consolidation (Figure 1A), nodular ground-glass opacities of the culmen, ventilatory disorders, and cardiomegaly (Figure 1B).
We retained the diagnosis of hypoxic acute respiratory failure with heart and kidney failure secondary to COVID-19 pneumonia on kidney transplant, based on clinical symptoms, a positive SARS-CoV-2 PCR nasal swab test, and lung lesions on a chest CT scan.
We immediately started intravenous antibiotic drugs to cover a possible bacterial infection, and we administered diuretics due to congestive heart failure. Unfractionated heparin and corticosteroids were added and we maintained the patient’s immunosuppressive medications.
Faced with worsening clinical signs, the patient was transferred to the Intensive Care Unit (ICU), where he died 3 weeks later with acute respiratory distress syndrome (ARDS) and heart and kidney failure.
PATIENT 2:
On April 10, 2022, a 79-year-old Gabonese man presented with 10 days of dry cough associated with intermittent fever, anorexia, and fatigue, reporting self-medication with antimalarials without improvement in symptoms.
The patient’s medical history included high blood pressure for 20 years, well controlled on antihypertensive drugs, diabetes mellitus on oral antidiabetics, and kidney transplant associated with immunosuppressive drugs (cyclosporine and mycophenolate mofetil) due to end-stage renal disease. He had his first kidney transplant in 2007 (transplant rejection) and the second in 2012.
He was admitted to the COVID Unit after the onset of non-bloody diarrhea and worsening dyspnea. Physical examination showed blood pressure 130/60 mmHg, heart rate 115 beats/min, temperature 39°C, and bilateral condensation syndrome. The oxygen saturation level was 90%, which then rapidly decreased to 85–89%.
His blood tests results were: white blood cells 10710/mm3, neutrophiles 9290/mm3, lymphocytes 660/mm3, Hb 10.9 g/dl, MCV 81.2 ft, hematocrit 35%, platelets 102 000/mm3 (Table 3), D-dimers 1840 ng/ml, prothrombin rate 70%, CRP 72 mg/dL, AST 66 UI/L, ALT 44 UI/L, gamma glutamyl transferase 318 UI/L, LDH 673 UI/L, total bilirubin 28 µmol/l, direct bilirubin 14 µmol/l, lactic-dehydrogenase 673 UI/L, blood glucose 5.66 mmol/l, urea 22.6 mmol/l, creatinine 286 µmol/l, and creatinine clearance 19 ml/min (Table 4). Serologies of HIV and hepatitis B and C were negative. A SARS-CoV-2 PCR test was positive and a malaria test was negative.
A chest X-ray showed poorly non-systematized diffuse opacities (Figure 2). A chest CT scan without injection of contrast medium showed more than 70% lung lesions (Figure 3). An axial slice in the parenchymal window showed large areas of ground-glass opacities, bilateral posterior subpleural consolidations of both lung bases (Figure 3A), “crazy paving” pattern associated with bronchiectasis, and arterial dilation (Figure 3B).
Based on these results, we retained the diagnosis of severe COVID-19 pneumonia with acute failure on kidney transplant.
Therapeutic management included oxygen therapy at 15 L/min, broad-spectrum antibiotics, corticosteroids, unfractionated heparin, and insulin therapy, and we maintained the patient’s immunosuppressive drugs.
The patient then was transferred to the ICU within 24 h because he was showing persistent signs of acute respiratory distress, where he received 3 sessions of non-invasive ventilation, not well tolerated. After 10 days of intensive care, the patient died with multi-system failure (pulmonary, cardiac, and renal).
Discussion
These 2 kidney transplant patients presented upon admission with severe COVID-19 pneumonia associated with acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), and heart failure. ARDS has already been reported in kidney transplant patients with severe COVID-19. Indeed, this was observed in the study by Bossini et al, who reported that of 45 patients admitted to the hospital with severe COVID-19 symptoms, 60% developed ARDS [7].
Apart from the pulmonary tropism of SARS-CoV-2, the kidney is also one of the main organs affected in severe COVID-19. The AKI observed in our 2 patients could be explained by several complex mechanisms, mainly the high level of expression of angiotensin-converting enzyme 2 (ACE2) receptors on renal epithelial cells on which the virus binds by using its Spike (S) protein, the direct cytotoxicity of SARS-CoV-2 on the kidney, and the systemic cytokine-induced inflammatory response, also called “cytokine storm”.
According to Adapa et al, AKI caused by COVID-19 affects kidney transplant recipients and seems to be an independent risk factor for mortality [8]. Additionally, Toapanta et al reported that kidney transplant patients of African descent with AKI have a higher risk of COVID-19 [4]. To reinforce this hypothesis, according to Nasr et al, Black race seems to increase the risk of kidney failure in kidney transplant recipients. This could be explained by the fact that in this target population, the Apolipoprotein L 1 (APOL1) genotype is closely linked to the development of nephropathy, and the evidence suggests that SARS-CoV-2 infection could act as a “trigger” leading to dys-regulation of podocytes [9]. The clinical signs observed were fever, diarrhea, and fatigue. These symptoms were also reported by Guillen et al and Abu Jawdeh et al [10,11], but these symptoms are non-specific and have also been described in the general population of COVID-19 patients [5,12].
In Chaudhry et al’s study, gastrointestinal symptoms such as diarrhea were common and were reported in 54.3% of solid organ transplant recipients compared to 17% of non-organ transplant patients. In this population, diarrhea could be a direct consequence of infection with SARS-CoV-2, which uses ACE2 for entry and the serine protease TMPRSS2 for protein priming; both are expressed in the epithelium of the small intestine [13].
The lymphopenia observed in our patients could be predictive of the severity of COVID-19 [14]. An increase D-dimers was reported in both patients. These results were in agreement with those of Chen et al, who observed a 7-fold increase in normal D-dimer values in patients with severe COVID-19 admitted to the hospital compared to those with moderate COVID-19 [15]. These results suggest that coagulopathy plays an important role in the pathophysiological mechanism of SARS-CoV-2 in COVID-19 patients. Furthermore, D-dimers as a biomarker could have important prognostic utility in COVID-19 [16]. Inflammatory biomarkers such as CRP, which was elevated in our 2 kidney transplant patients, could also be correlated with the severity of disease [17].
Tropical regions do not always have access to rapid SARS-CoV-2 PCR tests; therefore, while awaiting the results, the chest CT scan has been a very useful tool in the diagnosis of COVID-19 pneumonia and has allowed us to highlight the aspects of subpleural consolidations, ground-glass opacities, and “crazy paving” pattern suggestive of pneumonia. These lung abnormalities have also been described by Devresse et al [5].
Although there are no consensus guidelines on the management of kidney transplant recipients with COVID-19, the treatment protocol we used included oxygen therapy and antibiotics if bacterial superinfection was suspected. Dexamethasone and unfractionated heparin were used to reduce hyper-inflammation and coagulopathy related to SARS-CoV-2 [6].
We maintained use of immunosuppressive drugs because of the benefit/risk ratio with a potential risk of acute renal transplant rejection and the impossibility in our region of adapting therapy by serum dosage of these molecules, but this choice is not in agreement with Arpali et al and Johnson et al, who strongly recommend stopping antimetabolites (eg, mycophenolate mofetil, azathioprine) and reducing doses of calcineurin inhibitors (eg, tacrolimus, cyclosporine) or stopping them completely in severe cases of COVID-19 [18,19].
Our 2 patients died in a context of ARDS, heart failure, and renal failure. Indeed, according to Cravedi et al, kidney transplant recipients hospitalized with COVID-19 have a higher mortality rate (32%) due to chronic immunosuppression from kidney transplant [20]. However, other risk factors, including advanced age, high blood pressure, diabetes mellitus, and immunosuppressive drugs, observed in our patients may also contribute to the increased mortality rate [10,21].
Conclusions
These 2 cases show that severe forms of COVID-19 associated with acute renal failure, both with kidney transplantation, can be fatal. Pending SARS-CoV-2 PCR results, elevated D-dimer level and lung abnormalities seen on chest CT scan were important tools to guide our diagnosis of COVID-19 pneumonia. After 2 years of the pandemic, kidney transplant patients with COVID-19 appear to be at high risk of developing severe COVID-19 due to immunosuppression, especially in low-income countries. However, additional studies with larger samples are needed to better assess this risk in our region.
Figures
Tables
Table 1.. Patient 1: Complete blood count results at admission and flagged values.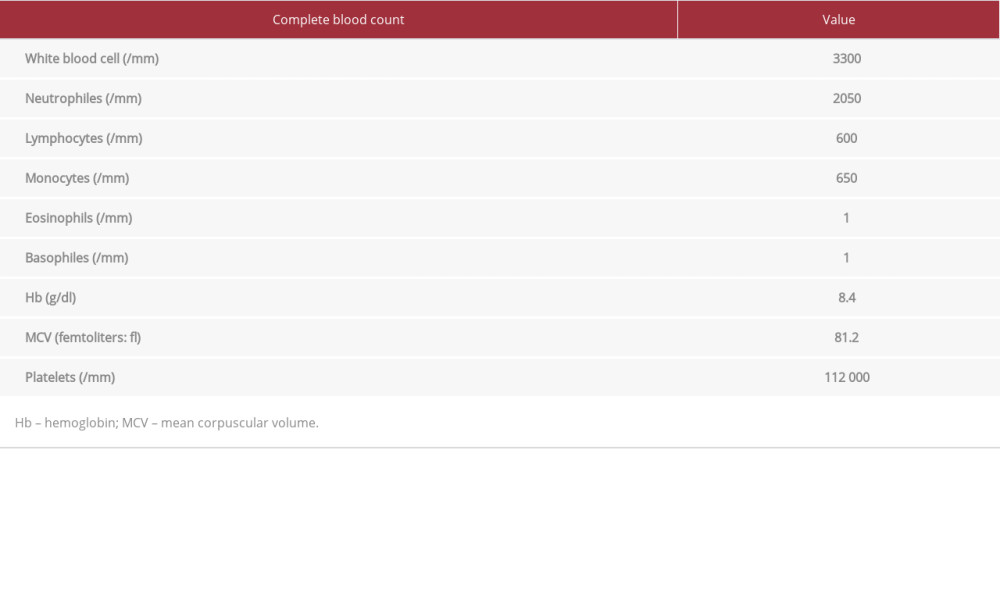 Table 2.. Patient 1: Biochemistry results at admission and values.
Table 2.. Patient 1: Biochemistry results at admission and values.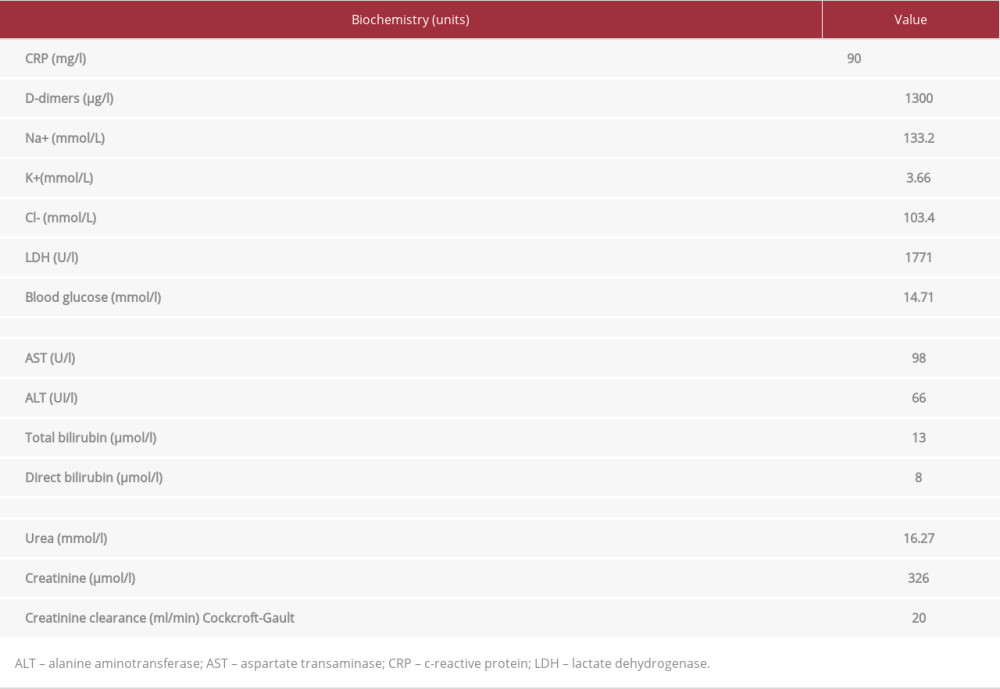 Table 3.. Patient 2: Complete blood count results at admission and flagged values.
Table 3.. Patient 2: Complete blood count results at admission and flagged values.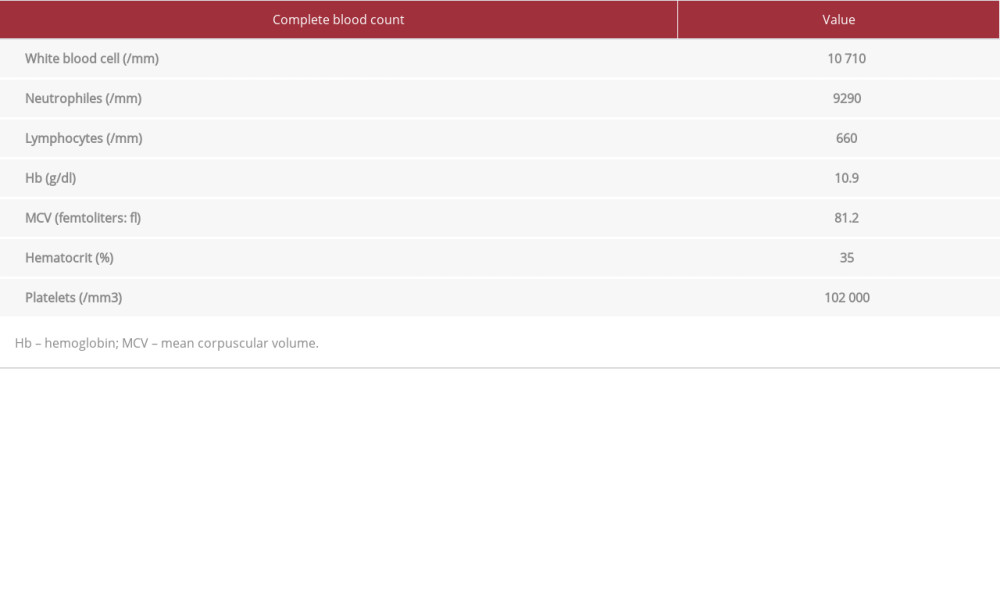 Table 4.. Patient 2: Biochemistry results at admission and values.
Table 4.. Patient 2: Biochemistry results at admission and values.
References:
1.. (Consulted the 28 May 2020)https://www.who.int/emergencies/diseases/novel-coronavirus-2019
2.. Iroungou BA, Mangouka LG, Bivigou-Mboumba B, Demographic and clinical characteristics associated with severity, clinical outcomes, and mortality of COVID-19 infection in Gabon: JAMA Netw Open, 2021; 4(9); e2124190
3.. Silva F, Cipriano A, Cruz H, SARS-CoV-2 infection in kidney transplant recipients: Early report of five cases: Transpl Infect Dis, 2021; 23(1); e13394
4.. Toapanta N, Torres IB, Sellares J, Kidney transplantation and COVID-19 renal and patient prognosis: Clin Kidney J, 2021; 14(Suppl. 1); i21-i29
5.. Devresse A, Belkhir L, Vo B, COVID-19 infection in kidney transplant recipients: A single-center case series of 22 cases from Belgium: Kidney Med, 2020; 2(4); 459-66
6.. McAdams M, Ostrosky-Frid M, Rajora N, Hedayati S, Effect of COVID-19 on kidney disease incidence and management: Kidney360, 2021; 2(1); 141-53
7.. Bossini N, Alberici F, Delbarba E, Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience: Am J Transplant, 2020; 20(11); 3019-29
8.. Adapa S, Chenna A, Balla M, COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation: J Clin Med Res, 2020; 12(6); 352-61
9.. Nasr SH, Kopp JB, COVID-19-associated collapsing glomerulopathy: An emerging entity: Kidney Int Rep, 2020; 5(6); 759-61
10.. Abu Jawdeh BG, COVID-19 in kidney transplantation: Outcomes, immuno-suppression management, and operational challenges: Adv Chronic Kidney Dis, 2020; 27(5); 383-89
11.. Guillen E, Pineiro GJ, Revuelta I, Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation?: Am J Transplant, 2020; 20(7); 1875-78
12.. Guan WJ, Ni ZY, Hu Y, Clinical characteristics of coronavirus disease 2019 in China: N Engl J Med, 2020; 382(18); 1708-20
13.. Chaudhry ZS, Williams JD, Vahia A, Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: A cohort study: Am J Transplant, 2020; 20(11); 3051-60
14.. Akalin E, Azzi Y, Bartash R, COVID-19 and kidney transplantation: N Engl J Med, 2020; 382(25); 2475-77
15.. Chen T, Wu D, Chen H, Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published correction appears in BMJ 2020;368: m1295]: BMJ, 2020; 368; m1091
16.. Zhou F, Yu T, Du R, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study: Lancet, 2020; 395(10229); 1054-62
17.. Fishman JA, The immunocompromised transplant recipient and SARS-CoV-2 infection: J Am Soc Nephrol, 2020; 31(6); 1147-49
18.. Arpali E, Akyollu B, Yelken B, Case report: A kidney transplant patient with mild COVID-19: Transpl Infect Dis, 2020; 22(4); e13296
19.. Johnson KM, Belfer JJ, Peterson GR, Managing COVID-19 in renal transplant recipients: A review of recent literature and case supporting corticosteroid-sparing immunosuppression: Pharmacotherapy, 2020; 40(6); 517-24
20.. Cravedi P, Mothi SS, Azzi Y, COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium: Am J Transplant, 2020; 20(11); 3140-48
21.. Elias M, Pievani D, Randoux C, COVID-19 infection in kidney transplant recipients: Disease incidence and clinical outcomes: J Am Soc Nephrol, 2020; 31(10); 2413-23
Figures
Tables
 Table 1.. Patient 1: Complete blood count results at admission and flagged values.
Table 1.. Patient 1: Complete blood count results at admission and flagged values. Table 2.. Patient 1: Biochemistry results at admission and values.
Table 2.. Patient 1: Biochemistry results at admission and values. Table 3.. Patient 2: Complete blood count results at admission and flagged values.
Table 3.. Patient 2: Complete blood count results at admission and flagged values. Table 4.. Patient 2: Biochemistry results at admission and values.
Table 4.. Patient 2: Biochemistry results at admission and values. Table 1.. Patient 1: Complete blood count results at admission and flagged values.
Table 1.. Patient 1: Complete blood count results at admission and flagged values. Table 2.. Patient 1: Biochemistry results at admission and values.
Table 2.. Patient 1: Biochemistry results at admission and values. Table 3.. Patient 2: Complete blood count results at admission and flagged values.
Table 3.. Patient 2: Complete blood count results at admission and flagged values. Table 4.. Patient 2: Biochemistry results at admission and values.
Table 4.. Patient 2: Biochemistry results at admission and values. In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








