05 January 2023: Articles 
Immune-Mediated Adverse Drug Reactions (IM-ARDs) in the Form of Drug-Induced Immune Thrombocytopenia and Cutaneous Adverse Drug Reactions (CARD) Due to Clindamycin in an Human Immunodeficieny Virus (HIV) Patient
Unknown etiology, Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Adverse events of drug therapy, Educational Purpose (only if useful for a systematic review or synthesis)
Nata Pratama Hardjo LugitoDOI: 10.12659/AJCR.938358
Am J Case Rep 2023; 24:e938358
Abstract
BACKGROUND: Many drugs have been reported to cause immune-mediated adverse drug reactions (IM-ADRs) in human immunodeficiency virus (HIV) patients; the most common is cutaneous adverse drug reaction (CADR). Immune thrombocytopenia purpura (ITP) is frequent in HIV patients, and it can be caused HIV, opportunistic infections, or drugs. Although drugs can cause immune thrombocytopenia, termed drug-induced immune thrombocytopenia (DIIT), there has been no study on DIIT in HIV patients.
CASE REPORT: A 33-year-old male patient was admitted to our hospital with pruritic skin lesion over the entire body, which started 7 days before. He was diagnosed with HIV infection, brain toxoplasmosis, and pulmonary tuberculosis 2 weeks before admission, and was given trimethoprim sulphamethoxazole, isoniazid, rifampicin, pyrazinamide, and ethambutol. Clindamycin was added 10 days before admission. Skin examination revealed generalized erythematous macules with palpable petechiae and purpura. The platelet count was 141 000/µL when he was diagnosed with HIV, and it was 2000/µL at the time of admission. Clindamycin was discontinued and he was given steroids and platelet transfusion. The skin lesions improved along with an increased platelet count. He was discharged on the 10th day of admission, with platelet count of 42 000/µL. When he returned to the outpatient clinic on the 15th day, his platelet was 54 000/µL. The skin lesions had resolved completely and become hyperpigmented, and no purpura or petechiae were seen.
CONCLUSIONS: We present a case of an HIV patient with IM-ADR in the form of DIIT in conjunction with CADR that might have been caused by clindamycin.
Keywords: clindamycin, HIV Infections, Purpura, Thrombocytopenic, Idiopathic, Male, Humans, Adult, Thrombocytopenia, Purpura, Drug-Related Side Effects and Adverse Reactions
Background
Adverse drug reaction in human immunodeficiency virus (HIV)/tuberculosis (TB)-endemic countries is a serious problem, accounting for 10% of admissions and 13% of deaths in 1 study [1]. Many drugs have been reported to cause immune-mediated adverse drug reactions (IM-ADRs) in HIV patients. The incidence of IM-ADRs is approximately 15% of HIV patients starting treatment, and the most common form is cutaneous adverse drug reaction (CADR). A significant portion IM-ADRs are severe cutaneous adverse drug reactions (SCAR), such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS). The 2 groups of drugs associated with IM-ADR-s are antiretrovirals and antiinfective agents for opportunistic infections. The most common are first-line anti-tuberculosis (TB) drugs, which are rifampicin, isoniazid, pyrazinamide, and ethambutol [1], and trimethoprim sulphamethoxazole [2,3]. There has been no report of clindamycin as a cause of IM-ADR in HIV patients. Immune thrombocytopenia purpura (ITP), especially in HIV patients, is common, and it can be caused primarily by HIV or secondarily by opportunistic infections, as well as by drugs. HIV patients often develop thrombocytopenia during the disease course [4]. There has been no study of drug-induced immune thrombocytopenia (DIIT) in HIV patients. To the best of our knowledge, there has been no report of CADR and DIIT in a patient. We report an HIV patient with DIIT in conjunction with CADR that might have been caused by clindamycin.
Case Report
A 33-year-old man was referred to our hospital with pruritic skin lesion over the entire body beginning 7 days before. The skin lesion started with red patches on the trunk, which later spread through the extremities. Two days before admission, there were also dark red pinpoint lesions on top of the red skin. There were no lesions in the mouth, eyes, or genitalia. He also experienced fever that began 5 days prior, but at admission his fever had resolved. Two weeks before admission, he had been diagnosed in another hospital with human immunodeficiency virus (HIV) infection, brain toxoplasmosis, and pulmonary tuberculosis. He was given trimethoprim sulphamethoxazole 960 mg b.i.d., gabapentin 300 mg t.i.d., folic acid 5 micrograms o.d., and the anti-tuberculosis drugs isoniazid 300 mg o.d., rifampicin 450 mg o.d., pyrazinamide 1000 mg o.d., and ethambutol 1000 mgs o.d. Ten days before admission, he was given clindamycin 600 mg q.i.d. for brain toxoplasmosis. Antiretrovirals had not been started. On physical examination, blood pressure was 110/70 mmHg, pulse 103 beats per minute, respiratory rate 18 breaths per minute, and temperature 37°C. General examination results were within normal limits. The skin examination found generalized erythematous macules and papules with palpable petechiae and purpura.
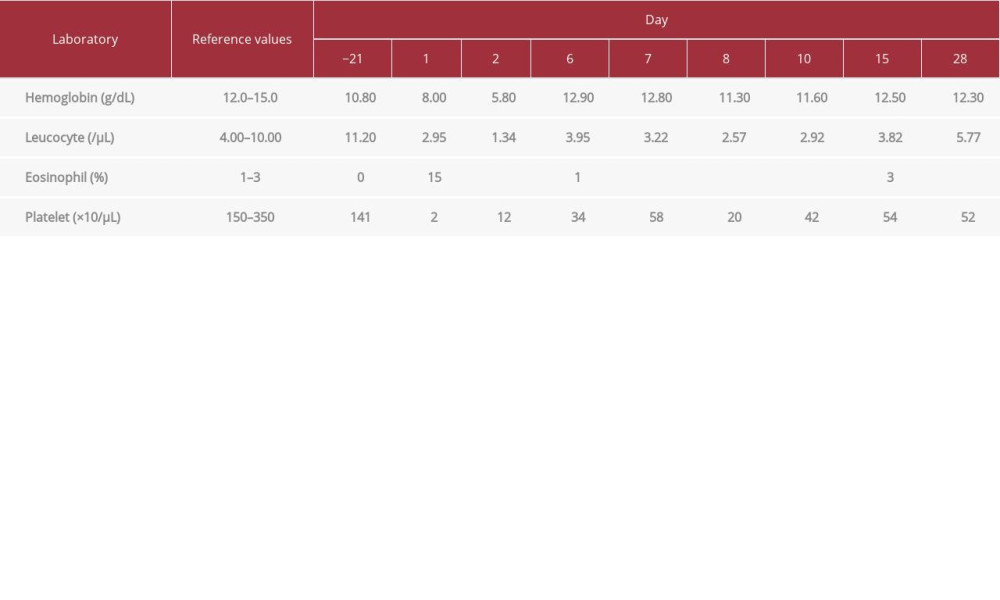
Laboratory examination 2 weeks before admission revealed hemoglobin 10.8 g/dL, hematocrit 32.0%, leucocytes 11 200/µL, differential count segment neutrophils 90% and lymphocytes 4%, platelets 141 000/µL, aspartate 36 U/L, alanine amino-transferase 23 U/L, urea 15.1 mg/dL, creatinine 0.63 mg/dL, uric acid 4.2 mg/dL, reactive HIV antibody, CD4 count 78/µL, negative Interferon-Gamma Release Assays, and anti-toxoplasma IgG 171 IU/mL. Contrast brain magnetic resonance imaging (MRI) showed enhanced multiple-ring lesions with irregular walls, the largest on the left basal ganglia (2.6×2.6×2.6 cm), and many smaller lesions on the cortex of the right frontal lobe, left parietal, right corona radiata, left occipital, left mesencephalon, left pons, and left cerebellum pedunculus. The lesions were accompanied by significant perifocal edema. MRS showed prominent increased lipid, with slightly increased choline and slightly decreased creatine. These results suggested an infection process of toxoplasmosis. Chest radiology revealed left pleuropneumonia.
Laboratory examination on the day of admission revealed hemoglobin 8.0 g/dL, hematocrit 22.3%, leucocytes 2950/µL, on differential count eosinophils 15%, segment neutrophils 73%, lymphocytes 4%, monocytes 5%, platelets 2000/µL, corrected reticulocyte count 1.32%, aspartate and alanine aminotransferase 31 U/L and 13 U/L, urea 13.0 mg/dL, creatinine 0.87 mg/dL, uric acid 12.0 mg/dL, CRP 17 mg/L, and IgE 381.9 IU/ml. Blood smear morphology showed normocytic normochromic anemia with anisopoikilocytosis and polychromasia, leucocyte count decreased with toxic granulation in the polymorphonuclear cells,and no blasts were found, platelet count was decreased, and no giant thrombocytes were found. He was HBsAg-negative and anti-HCV non-reactive, Antinuclear Antibodies (ANA) profile-negative (Anti-nRNP/SM 0; Anti-SM 1; Anti-SS-A 2; Anti-Ro-52 1; Anti-SS-B 2, Anti-Scl-70 1; PMScl 2; Anti-Jo-1 5; Anti-Centromeres 0; PCNA 2; Anti-ds-DNA 0; Anti-Nucleosome 0; Anti-Histone 1; Anti-Rib P-Protein 1; SAMA-M2 1; DFS70 1; control 60 (+++), C3 complement 105.0 mg/dL (normal range 90.0–180.0 mg/dL), C4 complement 23.0 mg/dL (normal range 15.0–53.0 mg/dL), and antineutrophil cytoplasmic antibodies (ANCA)-negative.
Pathological examination of the skin with hematoxylin-eosin staining revealed keratinized stratified squamous epithelium with a foci of flattened basement membrane on 40× magnification (Figure 1A). On 400× magnification, there were epidermis with spongiosis (white arrow) and dermis with stromal elastosis, and visible congestive blood vessel with mild lymphocytic inflammatory cells (black arrow). There was melanin incontinence, but there was no eosinophil or necrotic cells seen in the epidermis and dermis (Figure 1B).
The patient was given intravenous methylprednisolone 125 mg b.i.d. for 3 days, and then 62.5 mg b.i.d. for 3 days and 62.5 mg o.d. for 3 days. Clindamycin was stopped and other medications were continued. He was transfused with 10 units of thrombocyte concentrate on the first day of admission. On the second day of admission, the hemoglobin was 5.8 g/dL, platelets were 12 000/µL, and he was given 750 ml of packed red cells. On the third day of admission, the hemoglobin was 12.9 g/dL and platelets were 34 000/µL. The skin lesion had begun to resolve and the generalized erythematous macules became hyperpigmented, but palpable petechiae and purpura were still present.
The patient was discharged on the 10th day of admission, with hemoglobin 11.6 g/dL and platelets 42 000/µL. His medication consisted of oral methylprednisolone 16 mg t.i.d. for 7 days, and the same medications from admission. When he returned to the outpatient clinic on the 15th day, hemoglobin was 12.5 g/dL and platelets were 54 000/µL. The skin lesions had resolved completely and become hyperpigmented, and no purpura or petechiae were seen. The laboratory results are shown in Table 1. Figure 2 shows the development of skin lesions and Figure 3 shows the platelets curve count. The patient did not return for a follow-up appointment afterwards and could not be reached.
Discussion
The HIV patient in this case report had thrombocytopenia and CADR. Should thrombocytopenia in this HIV patient be considered as a distinct problem from CADR? Could the thrombocytopenia be considered as DIIT? If the thrombocytopenia was drug-induced, then it would be an entity of adverse drug reaction, together with CADR.
Drugs that cause immune-mediated adverse drug reactions (IM-ADRs) in HIV patients were divided into 2 groups: anti-retrovirals and antiinfective agents used to treat opportunistic infections. The most common are first-line anti-tuberculosis (TB) drugs, which are rifampicin, isoniazid, pyrazinamide, and ethambutol [1]. Trimethoprim sulphamethoxazole is also a cause of IM-ADR, especially DRESS, SJS/TEN, and fixed-drug eruption [2,3,5]. There has been no report of clindamycin as a cause of IM-ADR in HIV patients. ITP in HIV patients could be caused primarily by HIV or secondarily by opportunistic infections, and also drugs. Approximately 40% of HIV patients develop thrombocytopenia during the disease course [4].
The current knowledge of the IM-ADRs mechanism is limited, and the mechanism of increased susceptibility to drug reactions in HIV patients is also unclear. The mechanisms are thought to be multifactorial as an interaction between pre-HIV genetic risk, immunologic, metabolic, pharmacologic, HIV, and opportunistic infection in the course of HIV infection [1]. In HIV infection, the immune dysregulation due to depletion of immunoregulatory cells and increased oxidative stress due to consumption of antioxidative substances provides a cascade of cytokine release and hypersensitivity reaction [6].
The skin lesions of this HIV patient were described as generalized erythematous macules and papules with palpable pete-chiae and purpura, and they resembled morbilliform eruption, which represents 95% of all CADR. The morbilliform eruption appears 7–14 days after initiation of the suspected drug, and has a pruritic, symmetrical, erythematous, maculopapular eruption involving the trunk and intertriginous areas, but sparing the face [7]. Pathological examination shows a perivascular and dermal lymphocytic infiltrate, with occasional subtle vasculopathic features, and also mild accompanying lymphocytic exocytosis and spongiosis, but scattered eosinophilic leucocytes were not present in our patient [7]. Another presentation of CADR is vasculitis eruption, which has palpable purpura predominantly in the extremities, and even plaques, ulcers, and blisters. Pathology examination shows neutrophilic/leukocytoclastic vasculitis of postcapillary venules and extravasation of red blood cells, which were not found in our patient [8].
Another form of IM-ADRs is DRESS, which is a severe hyper-sensitivity reaction to a drug or its reactive metabolites, associated with enzymatic defects in drug metabolism [8]. The most common cause of DRESS are anticonvulsants (such as phenytoin, carbamazepine, and phenobarbital), and sulfonamides (such as dapsone and sulfasalazine). DRESS usually manifests 2 months after initiation of the drug, mostly in 2 to 6 weeks, involved multiple organs, especially skin, liver, and hematologic system [9]. The most common skin lesions in DRESS are facial edema and urticated maculopapular eruption, which were present in our patient, but it can also present as vesicle, bullae, pustule, cheilitis, purpura, target lesion, and erythroderma. Hematological manifestations are leukopenia or lymphopenia that precedes leukocytosis with atypical lymphocytes, and a marked eosinophilia in 30% of cases, as well as thrombocytopenia and anemia [7,10]. The diagnosis of DRESS syndrome is based on the RegiSCAR scoring system [11]. The RegiSCAR score of our patient was 1, and graded as “no” (fever >38.5°C – no (−1), enlarged lymph node – no (0), eosinophilia >10% of WBC – yes (1), atypical lymphocytosis – no (0), skin rash extent > 50% of BSA – yes (1), rash suggesting DRESS – yes (1), skin biopsy suggesting DRESS – no (−1), rash resolution >15 days – no (−1), and excluding other causes – yes (1). Pathology examination of DRESS shows dense perivascular lymphocytic infiltrate in the papillary dermis, with eosinophils, atypical lymphocytes, and spongiosis of the epidermis.
Immune thrombocytopenia purpura (ITP), especially in HIV patients, is common. Thrombocytopenia in HIV patients can be caused primarily by HIV or secondarily by opportunistic infections [4]. In primary thrombocytopenia, autoimmune destruction dominates in early stages of HIV, and defective thrombopoiesis dominates in the later stages [4]. In secondary thrombocytopenia, important causes are opportunistic infections, of which TB is the most common [12,13]. Our patient had no personal or family history of thrombocytopenia. Hepatitis B and C serology was non-reactive, but
There has been no study on DIIT in HIV patients, although there were some reports of immune thrombocytopenia in HIV patients associated with drugs, such as amphotericin B and trimethoprim sulphamethoxazole, rifampicin, lopinavir/ritonavir, and indinavir [15–17]. It is unclear whether the thrombocytopenia in these HIV patients was DIIT. It can take 5–7 days of drug exposure to acquire sensitization. Platelet counts can decrease rapidly from normal levels to the lowest of below 10 000/µL in several hours of repeated exposure [18]. Our patient had a nearly normal platelet count (141 000/µL) 21 days before admission, which decreased to very low (2000/µL) on the day of admission. The patient had taken trimethoprim sulphamethoxazole, gabapentin, folic acid, isoniazid, rifampicin, pyrazinamide, and ethambutol for 21 days, and clindamycin for 10 days. It is possible that isoniazid, rifampicin, pyrazinamide, and ethambutol caused DIIT in our patient, complicated by sepsis, which had further worsened his thrombocytopenia [15–17]. The qSOFA or SOFA score for sepsis was not calculated due to incomplete data, but using the Sepsis-3 criteria, he fulfilled the sepsis criteria: documented lung tuberculosis and brain toxoplasmosis plus the presence of at least one SIRS criteria including pulse 103 beats per minute and leucocyte 2950/µL on the day of admission in our hospital). The etiology of sepsis in this patient was likely tuberculosis and toxoplasmosis, as a study in Africa found that tuberculosis and cryptococcal infection are common causes of sepsis and death in HIV patients [19]. It is probable that pancytopenia in this patient was due to sepsis-mediated bone marrow suppression. It has been shown in many studies that sepsis-mediated suppression of bone marrow results in impaired hematopoietic progenitor cells and their supporting niche cells, which leads to anemia, leucopenia, and thrombocytopenia [20,21].
Trimethoprim sulphamethoxazole should also be considered as causes of thrombocytopenia [22]. Another possible cause of thrombocytopenia is clindamycin, because the very low platelet count appeared after 10 days of repeated exposure to clindamycin. A 2011–2012 study in the Platelet and Neutrophil Immunology Lab of the Blood Center of Wisconsin, Milwaukee showed that clindamycin had drug-dependent platelet antibodies (DDAbs) in patient sera [18]. The 5 clinical criteria to established the suspected drug in DIIT could not be applied as the patient had taken multiple drugs prior to the onset of thrombocytopenia.
We should also consider the possibility that thrombocytopenia in this patient was part of his pancytopenia, as he also had decreased hemoglobin level and leucocyte count. Drugs such as cytotoxics, chloramphenicol, idiosyncratic (immune-mediated), non-steroidal anti-inflammatory drugs, colchicine, sulfonamides, phenothiazines, thiazides, anti-thyroid drugs, anti-epileptics, and anti-diabetics could also be the cause of pancytopenia [22]. Our patient was treated with drugs that could cause pancytopenia, including trimethoprim-sulphamethoxazole and gabapentin. Sepsis and HIV alone could also have caused pancytopenia in our patient, as stated above. Considering clindamycin was the last drug given to the patient, it is very plausible that the thrombocytopenia and CADR were related to clindamycin.
The main management of drug-induced thrombocytopenia is discontinuation of the suspected drugs and managing symptoms of bleeding by platelet transfusions. Management of drug-induced thrombocytopenia using steroids has shown no benefit, but steroids could be given in severe thrombocytopenia such as in our case, where it is difficult to determine which of the multiple drugs was causing thrombocytopenia [23]. Management of CADR can also include discontinuation of suspected drugs. In the case of recurrent and potentially severe CADR, systemic steroids could be given [8]. This patient’s management was discontinuation of the suspected drug, which was clindamycin, administration of an immunosuppressive dosage of steroids, and platelet transfusion. The skin lesion of the patient improved along with the increased platelet count, as shown in Figures 2 and 3.
Conclusions
We report a case of an HIV patient with DIIT in conjunction with CADR that might have been caused by clindamycin. The improvement of skin lesion after clindamycin discontinuation and steroid therapy further supports our hypothesis. Regarding thrombocytopenia, although the platelet count improved, it did not return to normal level. It was very difficult to precisely pinpoint a single drug as the definite cause of thrombocytopenia and CADR in this patient, due to the multiple medication he was taking.
Figures
References:
1.. Petera J, Choshi P, Lehloenya RJ, Drug hypersensitivity in HIV infection: Curr Opin Allergy Clin Immunol, 2019; 19(4); 272-82
2.. Hiransuthikul A, Rattananupong T, Klaewsongkram J, Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS): 11 years retrospective study in Thailand: Allergol Int, 2016; 65; 432-38
3.. Kouotou EA, Nansseu JR, Ngono VN, Prevalence and clinical profile of drug eruptions among antiretroviral therapy-exposed HIV infected people in Yaounde, Cameroon: Dermatol Res Pract, 2017; 2017; 6216193
4.. Chandra N, Bhavnadhar P, Satyanarayana Raju Y, Shanthveer U, HIV-associated thrombocytopenia: J Clin Sci Res, 2012; 1; 187-91
5.. Mergler R, Chuang M, Stevens Johnson syndrome with vaginal pain and lesions as initial presentation: Am J Case Rep, 2018; 19; 1519-21
6.. Smith KJ, Skelton HG, Yeager J, Increased drug reactions in HIV-1-positive patients: a possible explanation based on patterns of immune dysregulation seen in HIV-1 disease. The Military Medical Consortium for the Advancement of Retroviral Research (MMCARR): Clin Exp Dermatol, 1997; 22; 118-23
7.. Grayson W, The HIV-positive skin biopsy: J Clin Pathol, 2008; 61(7); 802-17
8.. Hoosen K, Mosam A, Dlova NC, Grayson W, An update on adverse cutaneous drug reactions in HIV/AIDS: Dermatopathology, 2019; 6(2); 111-25
9.. De A, Rajagopalan M, Sarda A, Das S, Biswas P, Drug reaction with eosinophilia and systemic symptoms: An update and review of recent literature: Indian J Dermatol, 2018; 63; 30-40
10.. Husain Z, Reddy BY, Schwartz RA, DRESS syndrome: Part I. Clinical perspectives: J Am Acad Dermatol, 2013; 68; 693.e1-14
11.. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist?: Br J Dermatol, 2007; 156; 609-11
12.. Lambert MP, Gernsheimer TB, Clinical updates in adult immune thrombocytopenia: Blood, 2017; 129; 2829-35
13.. Babina S, Kumar TJ, Roy M, Konjenbam R, Thrombocytopenia in HIV/AIDS: IOSR J Dent Med Sci, 2015; 14; 9-11
14.. Krishna MR, Gottam US, Mahendra N, Disseminated tuberculosis with severe immune thrombocytopenia: Respir Med Case Rep, 2019; 27; 100812
15.. Camara-Lemarroy CR, Flores-Cantu H, Calderon-Hernandez HJ, Drug-induced haemolysis, renal failure, thrombocytopenia and lactic acidosis in patients with HIV and cryptococcal meningitis: A diagnostic challenge: Int J STD AIDS, 2015; 26(14); 1052-54
16.. Colebunders R, Schacht CD, Vanwolleghem T, Callens S, Lopinavir/ritonavir- and indinavir-induced thrombocytopenia in a patient with HIV infection: Int J Infect Dis, 2004; 8; 315-16
17.. Lugito NPH, Lorens JO, Kwenandar J, Kurniawan A, The dilemma in a case of immune thrombocytopenia in a patient with human immunodeficiency virus on antituberculosis treatment for miliary pulmonary tuberculosis: Oxf Med Case Rep, 2019; 11; 486-89
18.. Curtis BR, Drug-induced immune thrombocytopenia: incidence, clinical features, laboratory testing, and pathogenic mechanisms: Immunohematology, 2014; 30(2); 55-65
19.. Chaka W, Berger C, Huo S, Presentation and outcome of suspected sepsis in a high-HIV burden, high antiretroviral coverage setting: Int J Infect Dis, 2020; 96; 276-83
20.. Jiang Y, Jiang F-Q, Kong F, Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: A preliminary observational study: Ann. Intensive Care, 2019; 9; 67
21.. Yin F, Qian H, Duan C, Ning B, The bone marrow niche components are adversely affected in sepsis: Mol Biomed, 2020; 1; 10
22.. George JN, Aster RH, Drug-induced thrombocytopenia: Pathogenesis, evaluation, and management: Hematology Am Soc Hematol Educ Program, 2009; 153-58
23.. Butt MU, Jabri A, Elayi SC, Azithromycin-induced thrombocytopenia: A rare etiology of drug-induced immune thrombocytopenia: Case Rep Med, 2019; 2019; 6109831
Figures
In Press
04 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.941835
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943042
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250