23 April 2023: Articles 
Kidney Transplantation from a Deceased Donor with COVID-19 Ad26.COV2-S Vaccine-Induced Thrombotic Thrombocytopenia
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Rare disease, Rare coexistence of disease or pathology
Ivan Neretljak1ABCDEF, Franjo Jurenec12AB, Hrvoje Smojver1BCDEF*, Zeljka Jurekovic3BDEFDOI: 10.12659/AJCR.938730
Am J Case Rep 2023; 24:e938730
Abstract
BACKGROUND: Vaccine-induced thrombosis and thrombocytopenia is a rare immune disorder documented after adenoviral vector ChAdOx1 nCOV-19 (AstraZeneca) and Ad26.COV2-S (Janssen) vaccine administration against severe acute respiratory syndrome coronavirus 2. It is a rare adverse effect with an incidence of 1 case per 100 000 exposures. The disorder represents altered immune response with proliferation of antibodies that bind to platelet factor 4 (PF4), leading to formation of thrombi and consumptive coagulopathy. Thrombosis combined with thrombocytopenia generally occurs in the first month following vaccination and can lead to fatal outcome, even in young, previously healthy individuals. These young adults ultimately may become solid organ donors. The main concerns with vaccine-induced thrombosis and thrombocytopenia solid organ donors are anti-PF4 antibodies transmission potential, risk of early major graft thrombosis, and serious bleeding.
CASE REPORT: In our center, 2 kidney transplantations were performed from a single brain-dead vaccine-induced thrombosis and thrombocytopenia donor following Ad26.COV2-S COVID-19 (Janssen) vaccine in October 2021, which represents the first 2 cases of kidney transplantation from a deceased vaccine-induced thrombosis and thrombocytopenia donor after immunization with Ad26.COV2-S (Janssen) vaccine. Both recipients were closely monitored in the early post-transplantation period and after discharge from the hospital. To date, both recipients have a good functioning allograft, without any evidence of vaccine-induced thrombosis and thrombocytopenia transmission.
CONCLUSIONS: Our results are consistent with those of previously published cases of successful vaccine-induced thrombosis and thrombocytopenia donor solid organ transplantation. Kidney allografts transplanted from vaccine-induced thrombosis and thrombocytopenia donors can have a good overall function with favorable outcomes.
Keywords: Ad26.COV2.S Vaccine, COVID-19, Kidney Transplantation, young adult, Humans, COVID-19 Vaccines, Ad26COVS1, ChAdOx1 nCoV-19, Tissue Donors, Thrombocytopenia, Thrombosis
Background
Vaccine-induced thrombocytopenic thrombosis (VITT) after COVID-19 vaccination is a rare complication that has been reported for ChAdOx1 nCOV-19 (AstraZeneca) and Ad26.COV2-S (Janssen) vaccine [1,2]. It is similar to heparin-induced thrombocytopenia but without prior heparin exposure. It is also similar because heparin-induced thrombocytopenia antibodies, the antibodies directed against the platelet factor 4 (PF4) heparin complex, were identified. These antibodies activate platelets that lead to thrombosis and thrombocytopenia. The syndrome begins 3 to 30 days after vaccination and manifests with symptoms of thrombocytopenia (petechiae or mucosal bleeding) or thrombosis. Thrombosis has been the presenting feature in most of the initial reported cases [3,4]. Symptoms include deep vein thrombosis, pulmonary embolism, severe headache, abdominal pain, and chest pain. Intracerebral hemorrhage was also described as a presentation of thrombosis (cerebral venous thrombosis) [5].
Solid organ transplantation from a brain-dead VITT donor is feasible, but there are some considerations regarding early major thrombosis or significant bleeding and the possible transmission of anti-PF4 antibodies [6].
Here, we discuss 2 kidney transplantation procedures performed from a brain-dead VITT donor.
Case Reports
PATIENT 1: 65-YEAR-OLD WOMAN:
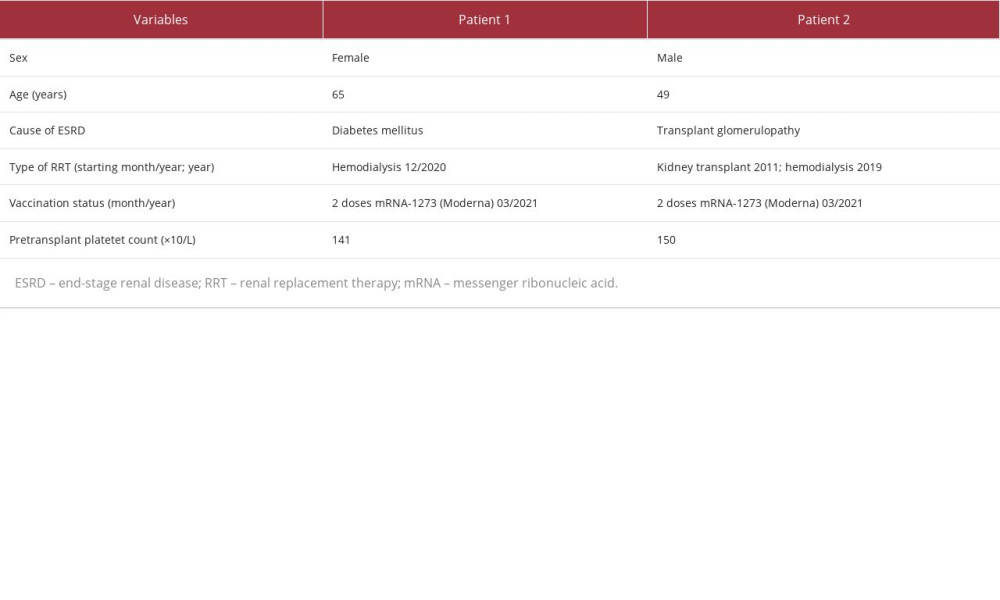
The cause of end-stage renal disease (ESRD) in this patient was diabetes. In December 2020, she started hemodialysis by an arteriovenous fistula. She was vaccinated with a 2-dose regimen of mRNA-1273 (Moderna) in March 2021 (Table 1).
A right Gibson incision was made, and the kidney was transplanted in the right iliac fossa. The vessels were sutured termino-laterally on the external iliac vein and artery using 6.0 Prolene suture. The anastomosis time was 30 min. A Lich-Gregoir ureterovesical anastomosis was made with the placement of a JJ stent.
The patient was of low immunological risk, with virtual panel reactive antibodies of 0%. Induction of immunosuppression started with basiliximab, and the maintenance of immunosuppression continued with a calcineurin inhibitor (tacrolimus), mycophenolate mofetil, and prednisolone.
Fondaparinux 2.5 mg (Arixtra®) was used instead of heparin for thromboprophylaxis.
The patient’s platelet count was 141×109/L prior to transplantation. No decreases in platelet count were observed during her hospital stay. Anti-PF4 antibodies were not tested after transplantation because there was no clinical indication. The primary function of the renal graft had been established. On postoperative day 4, the patient had macrohematuria, without laboratory signs of coagulopathy or a decrease in platelet count.
The CT scan revealed blood clots within the graft’s renal pelvis, without hydronephrosis or any other lesion. The macrohematuria stopped spontaneously. Due to the rise of creatinine levels on the day 5, hemodialysis was performed. After a single hemodialysis, normal graft function was established. During follow-up, no intrapyelic blood clots were seen by ultrasound. Upon establishment of complete recovery of graft function after the single hemodialysis, graft biopsy was not performed at this moment because there was no clinical indication. The patient was discharged from the hospital on postoperative day 11, with a creatinine serum level of 104 µmol/L; glomerular filtration rate (GFR) of 46.1 mL4in/1.73 m2 (MDRD).
A later allograft protocol biopsy, at 2 months after transplantation, revealed acute T cell-mediated rejection, Banff grade IB, successfully treated with pulsed intravenous methylpredniso-lone 500 mg administered for 3 consecutive days. Four months after transplantation, the creatinine level was 98 µmol/L.
PATIENT 2: 49-YEAR-OLD MAN:
The cause of ESRD in this patient was transplant glomerulopathy, probably due to chronic antibody-mediated rejection (no biopsy available). He was vaccinated with a 2-dose regimen of mRNA-1273 (Moderna) in April 2021.
In 2011, the patient received his first kidney graft. In 2019, after graft failure, he started hemodialysis. The patient was of increased immunological risk, with virtual panel reactive antibodies of 38%, but without the presence of donor-specific antibodies. The surgical technique, thromboprophylaxis, and immunosuppression were the same as for the first patient, except the left kidney was placed in the right iliac fossa. Induction of immunosuppression with anti-thymocyte globulin or rituximab was not performed owing to the epidemiological situation with COVID-19 at the moment, which was the preference of our transplant center during the pandemic. Induction of immunosuppression started with basiliximab, and the maintenance of immunosuppression continued with a calcineurin inhibitor (tacrolimus), mycophenolate mofetil, and prednisolone.
A graft biopsy was performed before transplantation, with findings of glomeruli of normal morphology, moderate acute tubular damage, arterioles and arteries of normal morphology, and no signs of thrombosis.
Fondaparinux 2.5 mg (Arixtra®) was used instead of heparin for thromboprophylaxis.
The patient’s platelet count was 150×109/L prior to transplantation. No decrease in platelet count was observed during the hospital stay.
Postoperative care proceeded uneventfully, and the patient was discharged from the hospital on postoperative day 8, with the creatinine serum level of 118 µmol/L; GFR of 56.9 mL/min/1.73 m2 (MDRD). The further post-transplantation course proceeded without graft rejection. A later allograft protocol biopsy, at 2 months after transplantation, revealed glomeruli and arterioles of normal morphology, without signs of capillaritis, indicating a normal histological finding.
The serum creatinine level at the last visit was 104 µmol/L; GFR of 65.8 mL/min/1.73 m2 (MDRD).
In regard to kidney allografts transplanted from VITT donors, no specific antithrombotic protocol was used to decrease the possibility of postoperative graft thrombosis in these 2 recipients. The specific procedures related to VITT donors included fondaparinux administration instead of heparin, combined with daily platelet count assessment. Testing for the anti-PF4 antibodies in the recipients was not conducted owing to the inability of the laboratory to perform this specific test at that time.
Discussion
VITT is a rare medical condition preceded by ChAdOx1 nCOV-19 (AstraZeneca) and Ad26.COV2-S (Janssen) vaccination [7,8]. The incidence rate is 1 case per 100 000 exposures. Post-vaccination synthetized VITT antibodies bind to a similar site on PF4, thus facilitating PF4 clustering and formation of immune complexes, which leads to Fcγ receptor IIa (FcγRIIa; CD32a)-dependent platelet activation, resulting in thrombosis [9].
According to the UK Donor VITT Transplant Study Group data, 15 kidney-only and 1 simultaneous pancreas and kidney, along with 7 liver, 1 heart, 1 bilateral lung, and 1 pancreatic islet transplantations were performed from deceased donors diagnosed with VITT after ChAdOx1 nCoV-19 (AstraZeneca) immunization. Organ donors were predominantly young females (85%), with a median age of 34 years, who succumbed to intracranial hemorrhage in 92% of the cases. Of those, 54% died from cerebral venous sinus thrombosis, while 46% had extra-cranial thrombosis, including portal vein, splenic vein, mesenteric vein, and aortal thrombosis, and pulmonary embolism. The median time from vaccine administration to hospital admission was 10 days. Four kidney-only or simultaneous pancreas and kidney recipients experienced major postoperative complications in the first 9 days after transplantation, consisting of bleeds and arterial or venous allograft thromboses. Of those, 3 recipients had a major hemorrhage, while 1 recipient developed thrombosis/thromboembolism. Some recipients experienced more than 1 complication. Two kidney allograft recipients had delayed graft function requiring hemodialysis, and 1 kidney recipient required emergent explantation, owing to early allograft failure. After a 19-day follow-up period, 78% of all allografts had a well-preserved function. Among the patients who were tested for anti-PF4 antibodies, 3 to 22 days after transplantation, 6 kidney recipients maintained undetectable antibody levels, while 3 of 7 liver recipients tested showed detectable anti-PF4 antibody levels, with 1 recipient experiencing a thrombotic complication without allograft loss. These results are indicative of passive donor anti-PF4 antibody/pathogenic lymphocyte transmission potential or anti-PF4 antibody formation in allograft recipients after transplantation [6,8].
Loupy et al identified 5 potential deceased donors with VITT after ChAdOx1 nCoV-19 (AstraZeneca) immunization. The organs from 2 potential donors were discarded; one was because of family refusal and the other due to severe disseminated intravascular coagulation. The donors were 2 men and 1 woman who died from cerebral venous sinus thrombosis and intracranial hemorrhage. The median time from vaccine administration to ICU admission was 13 days. Six kidneys, along with 2 hearts, 1 liver, and 1 lung from 3 donors were recovered and transplanted to 9 allograft recipients. There were no severe thrombotic or major hemorrhagic events among kidney allograft recipients. In the early postoperative period, 2 kidney allograft recipients experienced delayed graft function. One revealed glomerular microthrombi at the kidney preimplantation biopsy and the other at the graft biopsy 10 days after transplantation, resulting in mild interstitial inflammation and tubulitis, which were successfully treated with steroid pulse therapy. Both recipients’ graft function recovered gradually over time. After a median follow-up period of 52 days, all allografts were functioning adequately. At a median follow-up of 10 days after transplantation, no allograft recipients had detectable rates of anti-PF4 antibodies. None of the recipients experienced de novo thrombocytopenia, and no transmission of VITT in allograft recipients occurred [8,10].
Study data within the Eurotransplant region identified 6 VITT donors who provided 20 organs to 17 recipients. Five donors received the ChAdOx1 nCoV-19 (AstraZeneca) vaccine. Only 1 donor received the Ad26.COV2-S (Janssen) vaccine, but no organ was transplanted from that donor. The donors were equally represented by sex, with a median age of 48 years, and they died from intracranial hemorrhage (67%) and cerebrovascular accidents. The median time from vaccine administration to ICU admission was 13 days. There were 9 kidney allograft recipients. One kidney allograft recipient experienced thrombotic microangiopathy, and recovered completely. In 1 split-liver female recipient, thrombosis of various vessels occurred, followed by liver cell necrosis, resulting in urgent retransplantation. These 2 complications in allograft recipients represent 11.8% of thrombosis-related events. After the median follow-up time of 43 days, 95% of allografts were functioning adequately. All kidney recipients were alive, with good allograft function [7].
These are the first 2 cases of kidney transplantation from a deceased VITT donor after immunization with Ad26.COV2-S (Janssen) vaccine. Both recipients had a good functioning allograft after a follow-up of 5 months. Thrombotic microangiopathy was excluded before implantation biopsy. There was no evidence of VITT transmission to the recipients.
Our results are consistent with those of previously published studies regarding VITT donors immunized with ChAdOx1 nCoV-19 (AstraZeneca) vaccine, presented by the UK Donor VITT Transplant Study Group, Loupy et al, and the Eurotransplant Region Group [6–10].
The literature for VITT after COVID-19 vaccine is sparse. Despite that, kidneys from deceased VITT donors can be accepted, considering there is a decrease in donor number during the COVID-19 pandemic and, consequently, in the number of transplantations performed. VITT donors are usually younger adults, mostly healthy or without severe comorbidities, deceased from central nervous sinus thrombosis or intracranial hemorrhage, and with a short length of hospital stay, thus with reduced risk for nosocomial infections – all of which makes them desirable organ donors. A special emphasis of transplant centers, both donor and recipient, should be placed upon detection of microvascular thrombosis in potential organ donors, preferably by organ biopsy prior to transplantation. Respecting these recommendations and according to the available literature, VITT donor kidney transplantation has a great rate of success. Nonetheless, recipients should be closely monitored for clinical signs and laboratory findings indicative of VITT. Clinical signs include headache, confusion, blurred vision, weakness on 1 side, leg swelling, severe abdominal or chest pain, upset stomach, bloody diarrhea, or anything unusual and severe. Laboratory findings consist of platelet counts and fibrinogen, D-dimer, and anti-PF4 antibody levels.
Conclusions
Kidney allografts transplanted from VITT donors can have a good overall function with favorable outcomes. Careful evaluation of the donor, the exclusion of thrombotic microangiopathy, and the risk and benefit assessment of the recipient are required for a successful VITT donor organ transplantation.
References:
1.. Greinacher A, Thiele T, Warkentin TE, Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination: N Engl J Med, 2021; 384(22); 2092-101
2.. , Joint CDC FDA Statement on Johnson & Johnson COVID-19 vaccine [Internet], 2021 Available from: https://www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.html
3.. Pishko A, Cuker A, Thrombosis after vaccination with messenger RNA-1273: Is this vaccine-induced thrombosis and thrombocytopenia or thrombosis with thrombocytopenia syndrome?: Ann Intern Med, 2021; 174(10); 1468-69
4.. Scully M, Singh D, Lown R, Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination: N Engl J Med, 2021; 384(23); 2202-11
5.. Palaiodimou L, Stefanou M, Katsanos A, Cerebral venous sinus thrombosis and thrombotic events after vector-based COVID-19 vaccines: Neurology, 2021; 97(21); e2136-e47
6.. Greenhall G, Ushiro-Lumb I, Pavord S, Organ transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia: Am J Transplant, 2021; 21(12); 4095-97
7.. van Bruchem M, van Rosmalen M, Warmerdam A, Outcome after organ transplantation from brain-dead donors after a cerebral insult following SARS-CoV-2 vaccination within the Eurotransplant Region: Transplantation, 2021; 106(1); e100-e2
8.. Wolfe C, Humar A, Buyer beware: The risks of donor-derived vaccine-induced thrombosis and thrombocytopenia: Am J Transplant, 2021; 21(12); 3829-30
9.. Huynh A, Kelton J, Arnold D, Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia: Nature, 2021; 596(7873); 565-69
10.. Loupy A, Goutaudier V, Jacquelinet C, Kerbaul F, Solid organ procurement and transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia: Am J Transplant, 2021; 21(12); 4098-101
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250