23 January 2023: Articles 
Central Diabetes Insipidus in the Background of Lithium Use: Consider Central Causes Despite Nephrogenic as the Most Common
Unusual clinical course, Unexpected drug reaction
Jeffrey J. Li1ABCDEF*, Shirley Tan1BCDEF, Takumi KawashitaDOI: 10.12659/AJCR.939034
Am J Case Rep 2023; 24:e939034
Abstract
BACKGROUND: Nephrogenic diabetes insipidus is a well-known adverse effect of lithium use. Albeit rare, there have also been documented cases of central diabetes insipidus (CDI) associated with lithium use.
CASE REPORT: A 31-year-old woman with a past medical history of bipolar disorder, managed with lithium 300 mg by mouth every day for 3 years, was assessed for a 1-year history of polyuria with accompanying polydipsia. During her initial hospital stay, her estimated urine output was more than 4 L per day. Initial labs showed elevated serum sodium (149 mmol/L; reference range 135-145), elevated serum osmolality (304 mOsm/kg; reference range 275-295), urine osmolality of 99 mOsm/kg (reference range 50-1200), and urine specific gravity (1.005; reference range 1.005-1.030). Lithium was at a subtherapeutic level of 0.05 mEq/L (reference range 0.6-1.2). Magnetic resonance imaging of the brain revealed no abnormalities of the pituitary gland. Two different occasions of desmopressin administration resulted in >50% increase in urine osmolality, confirming the diagnosis of CDI. Common causes of CDI, including trauma, tumors, and familial CDI, were ruled out and chronic lithium use was determined as the most probable cause for the patient’s CDI.
CONCLUSIONS: CDI in the background of chronic lithium use is rarely reported. We present this case to consider CDI as a differential diagnosis when evaluating polyuria and hypernatremia in patients with long-term lithium use. These presentations warrant the consideration of both types of diabetes insipidus in the differential diagnoses.
Keywords: Hypernatremia, Central Diabetes Insipidus, Lithium Carbonate, Arginine Vasopressin Deficiency, Female, Humans, Adult, Diabetes Insipidus, Neurogenic, Lithium, Polyuria, Diabetes Insipidus, Nephrogenic, Hypernatremia, Diabetes Mellitus
Background
The first-line treatment for bipolar disorder is lithium [1]. Long-term lithium use has been associated with various adverse effects, including cerebellar dysfunction, gastrointestinal distress, and diabetes insipidus [2]. Nephrogenic diabetes insipidus is known to occur in up to 20% to 40% percent of long-term lithium users and is more common than central diabetes insipidus (CDI) [3,4]. Central diabetes insipidus due to lithium use, however, has rarely been reported [5–8]. CDI recently had its name changed to “arginine vasopressin deficiency” [9] and is often caused by damage to the posterior pituitary gland, impairing the secretion of antidiuretic hormone (ADH) [10]. Other recently learned causes of CDI were from COVID-19 infection or from the COVID-19 mRNA vaccine [11,12]. We present the case of a patient who presented with onset of CDI in the background of long-term lithium use in the treatment of bipolar disorder.
Case Report
A 31-year-old woman with a past medical history of bipolar disorder, managed with lithium 300 mg by mouth every day for 3 years, presented to the hospital with a 1-year history of polyuria with accompanying polydipsia. Following admission and initial workup, the patient was also diagnosed with Guillain-Barre syndrome (GBS) with onset of weakness symptoms within the previous week; it was determined that her GBS and polyuria were separate, unrelated diagnoses. During her initial hospital stay, her estimated urine output was more than 4 L per day, indicating polyuria. The patient was not on any angiotensin-converting enzyme (ACE) inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), or thiazide or loop diuretics that can cause lithium toxicity [13]. Her past medical history was significant for hemorrhoids and her family history was unremarkable. She had markedly decreased oral intake for the past 3 months because of fear of hemorrhoid pain upon defecation.
On the initial encounter, vital signs were within normal limits with blood pressure of 118/63 mmHg, heart rate of 86 beats per minute, respiratory rate of 19 breaths per minute, oxygen saturation of 97%, and temperature of 36.5°C. Her body mass index was 41.2 kg/m2. Physical examination revealed cracked lips and dry mucosa.
Initial labs showed elevated serum sodium (149 mmol/L; reference range 135–145), elevated serum osmolality (304 mOsm/kg; reference range 275–295), urine osmolality of 99 mOsm/kg (reference range 50–1200), and urine specific gravity (1.005; reference range 1.005–1.030). Kidney function was normal with creatinine at 0.62 mg/dL (reference range 0.6–1.2) and eGFR at 122 mL/min (reference range >90). Lithium was at a subtherapeutic level of 0.05 mEq/L (reference range 0.6–1.2). Her thyroid stimulating hormone, serum calcium, glucose, and liver enzymes were unremarkable. Complete blood count was significant for macrocytosis (mean corpuscular volume 118 fL; reference range 80–100 fL) and slight anemia (hemoglobin 11.3 g/dL; reference range 12–16 g/dL), which were attributed to her malnutrition. Inflammatory enzymes were slightly elevated with C-reactive protein (CRP) at 0.94 mg/dL (reference range <0.50 mg/dL) and erythrocyte sedimentation rate (ESR) at 37 mm/h (reference range 0–20), which were attributed to her GBS. Magnetic resonance imaging (MRI) of the brain revealed no expansive processes, hypophysitis, loss of signal from the posterior pituitary, or otherwise any abnormalities of the pituitary gland (Figure 1).
Within a few days of admission, the patient’s sodium increased from 149 mmol/L to 151–154 mmol/L. It was then brought down to 145 mmol/L via 5% dextrose infusion. However, her sodium levels spiked back up to 149 mmol/L (Figure 2A). A desmopressin trial of 4 µg 1-deamino-8-d-arginine vasopressin (DDAVP), a synthetic form of desmopressin, was then administered subcutaneously without the need for water deprivation because of her already hypoosmotic urine (<100 mOsm/kg), hyperosmotic serum (≥295 mOsm/kg), and hypernatremic (>145 mmol/L) states. Her beginning urine osmolality was 74 mOsm/kg and peaked at 254 mOsm/kg within a 2-h time period after administration of desmopressin. Her hypernatremia also resolved, dropping from 149 mmol/L to 140 mmol/L the next day (Figure 2A). A repeat desmopressin trial (1 µg DDAVP subcutaneously) was given after water deprivation a week later. Before water deprivation, her urine osmolality was 145 mOsm/kg. After water deprivation for 10 h, her urine osmolality was 194 mOsm/kg. Following administration of desmopressin, her urine osmolality increased from 194 mOsm/kg to 362 mOsm/kg (Table 1). Copeptin and ADH levels were not measured as these assays were not available as in-house tests.
Discussion
The goal of water deprivation in the testing of diabetes insipidus was to achieve maximal ADH secretion, indicated by sodium level >145 mmol/L and serum osmolality ≥295 mOsm/kg [14]. Our patient during the initial trial already met the criteria for maximal ADH secretion (sodium 149 mmol/L and serum osmolality 295 mOsm/kg) and did not require further water deprivation before desmopressin administration. Following hypernatremia (>145 mmol/L) and hyperosmolar serum (≥295 mOsm/kg) levels, along with urine osmolality <300 mOsm/kg, administration of 1–4 µg desmopressin that results in >50% increase in urine osmolality is diagnostic for CDI; if there is <50% increase in urine osmolality, the result is diagnostic of nephrogenic DI [15]. Because our patient had an increase in urine osmolality by more than 50% (73 mOsm/kg to 254 mOsm/kg and 194 mOsm/kg to 362 mOsm/kg) within 2 h after desmopressin trials, her condition was diagnostic for CDI.
Before we went ahead with testing for diabetes insipidus, we considered other differentials for her polyuria. Psychogenic polydipsia may occur with bipolar disorder, but we ruled this out because her sodium level was >135 mmol/L; treatment would have been limiting water intake [16]. Hypercalcemia may also lead to polyuria, but we ruled that out as her calcium levels were normal; treatment would have been intravenous normal saline [17]. After the desmopressin trials, we ruled out nephrogenic diabetes insipidus because of a >50% increase in urine osmolality after DDAVP administration; treatment would have been amiloride and thiazide diuretics [18].
After we diagnosed her CDI, we first sought out possible causes for her CDI as there are very few case reports describing lithium-induced CDI [5–8]. Common causes of CDI include family history, craniopharyngiomas, germ cell tumors, trauma, and inflammatory pathologies [10]. We ruled out familial CDI because inheritance would have been in an autosomal dominant manner, and the patient had no relevant family history. We ruled out trauma, tumors, or any other insults to the pituitary gland or stalk because TSH levels were normal, and MRI of the pituitary gland and stalk showed no abnormalities (Figure 1). An unknown, underlying inflammatory pathology may be a possible cause for her CDI as her CRP and ESR were slightly elevated, but unlikely as the inflammation in her blood can be explained by her diagnosed GBS. Although GBS can be a rare trigger for CDI [19], we ruled out GBS as an etiology because her development of GBS was within the last month while her polyuria developed in the past year. Furthermore, the patient endorsed her polyuria had remained the same even after GBS diagnosis and treatment, strongly indicating that GBS did not contribute to her CDI. CDI due to inflammatory pathologies is also often characterized by pituitary stalk thickening on T1-weighted MRI [20], which was not seen on our patient’s MRI (Figure 1). If there were lesions to the posterior pituitary gland from tumors, trauma, or inflammatory pathologies, then there would have also been a disappearance of a bright signal in the posterior pituitary gland on T1-weighted MRI, indicating loss of ADH storage [21]. A possible explanation for the visible bright spot in our patient’s posterior pituitary gland would be the inhibition of ADH exocytosis from the gland. Thus, given the onset of this disease without a better explanation, we considered long-term lithium use as the most likely cause for our patient’s CDI. As described in Posner and Mokrzychi, their patient was on lithium for 3 years and was diagnosed with CDI after a water deprivation test, which was similar in presentation to our patient [5].
The mechanism for lithium-induced nephrogenic diabetes insipidus is understood as the downregulation of aquaporin channels in principal cells from lithium inhibiting cyclic adenosine monophosphate (cAMP) formation [22]. The mechanism for CDI is understood as either a lack of ADH synthesis in the supraoptic nuclei of the hypothalamus or impaired exocytosis of ADH from the posterior pituitary gland [23]. Exocytosis of vesicles containing ADH relies on the formation of cAMP [24]. Because lithium inhibited cAMP formation in the renal tubules, a potential explanation for lithium causing CDI would be the selective inhibition of cAMP formation in the posterior pituitary gland, preventing ADH vesicle exocytosis [25].
Current understanding of the interactions between lithium and posterior pituitary structures is limited. Despite knowledge about the mechanism of lithium-induced nephrogenic diabetes insipidus, more research is needed to understand the relationship between lithium and CDI, as the current literature only described 4 occurrences of CDI in the presence of lithium. Diabetes insipidus affects 1 in 25 000 people [26], but the concomitant occurrence of central and nephrogenic diabetes insipidus has yet to be revealed.
A limitation of our report is the lack of ADH or copeptin levels due to the unavailability of these tests at our hospital. ADH or copeptin may be used as another confirmatory test for CDI [14]. However, our patient’s more than 50% increase in urine osmolality after 2 different occasions of DDAVP administration was clearly diagnostic for CDI [15]. Another factor to consider was that our patient’s CDI had not been resolved. At the time of this report, the patient’s lithium had been discontinued for 3 weeks and was not restarted, but previous reports had shown that CDI resolved after lithium was discontinued for months [5]. Our patient’s CDI hypernatremia was managed well with DDAVP injections given as needed to control her sodium levels. We also added 5% dextrose to help control her sodium levels as needed; additionally, oral water intake was encouraged whenever the patient was thirsty. The patient’s sodium was subsequently normalized to 138–146 mmol/L following the above interventions, and urine output decreased from >4000 mL/day to 2500–3600 mL/day when desmopressin was given (Figure 2B). Prior to discharge, she was switched to daily oral DDAVP. During her hospital stay, our patient’s polyuria was not a primary concern to manage as she had unlimited access to the urinary catheter.
Conclusions
CDI in the background of chronic lithium use is a rare phenomenon. By obtaining a thorough history, performing a detailed physical examination, and obtaining relevant imaging studies, we ruled out other causes of CDI in our patient, thus leaving lithium as the most likely explanation. Ultimately, more research is needed to truly understand how lithium can cause impairment of ADH synthesis or secretion. Despite this, we suggest considering CDI in addition to nephrogenic diabetes insipidus in the investigation of polyuria and hypernatremia in patients who present with long-term lithium use.
Figures
References:
1.. Volkmann C, Bschor T, Köhler S, Lithium treatment over the lifespan in bipolar disorders: Front Psychiatry, 2020; 11; 377
2.. Hedya SA, Avula A, Swoboda HD, Lithium toxicity: StatPearls, 2022, StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK499992/
3.. Boton R, Gaviria M, Batlle DC, Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy: Am J Kidney Dis, 1987; 10(5); 329-45
4.. Grünfeld J-P, Rossier BC, Lithium nephrotoxicity revisited: Nat Rev Nephrol, 2009; 5(5); 270-76
5.. Posner L, Mokrzycki MH, Transient central diabetes insipidus in the setting of underlying chronic nephrogenic diabetes insipidus associated with lithium use: Am J Nephrol, 1996; 16(4); 339-43
6.. Baylis PH, Heath DA, Water disturbances in patients treated with oral lithium carbonate: Ann Intern Med, 1978; 88(5); 607-9
7.. Forrest JN, Cohen AD, Torretti J, On the mechanism of lithium-induced diabetes insipidus in man and the rat: J Clin Invest, 1974; 53(4); 1115-23
8.. Singer I, Rotenberg D, Puschett JB, Lithium-induced nephrogenic diabetes insipidus: In vivo and in vitro studies: J Clin Invest, 1972; 51(5); 1081-91
9.. Arima H, Cheetham T, Christ-Crain M, Changing the name of diabetes insipidus: A position statement of The Working Group for Renaming Diabetes Insipidus: Endocr Connect, 2022; 11(11); e220378
10.. Arima H, Azuma Y, Morishita Y, Hagiwara D, Central diabetes insipidus: Nagoya J Med Sci, 2016; 78(4); 349-58
11.. Rajevac H, Bachan M, Khan Z, Diabetes insipidus as a symptom of COVID-19 infection: Case report: Chest, 2020; 158(4); A2576
12.. Bouça B, Roldão M, Bogalho P, Central diabetes insipidus following immunization with BNT162b2 mRNA COVID-19 vaccine: A case report: Front Endocrinol (Lausanne), 2022; 13; 889074
13.. Juurlink DN, Mamdani MM, Kopp A, Drug-induced lithium toxicity in the elderly: A population-based study: J Am Geriatr Soc, 2004; 52(5); 794-98
14.. de Fost M, Oussaada SM, Endert E, The water deprivation test and a potential role for the arginine vasopressin precursor copeptin to differentiate diabetes insipidus from primary polydipsia: Endocr Connect, 2015; 4(2); 86-91
15.. Gubbi S, Hannah-Shmouni F, Koch CA, Verbalis JG, Diagnostic testing for diabetes insipidus: Endotext, 2022, MDText.com, Inc. https://www.ncbi.nlm.nih.gov/books/NBK537591/
16.. Kotagiri R, Kutti Sridharan G, Primary polydipsia: StatPearls, 2020, StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK562251/
17.. Sadiq NM, Naganathan S, Badireddy M, Hypercalcemia: StatPearls, 2022, StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK430714/
18.. Hui C, Radbel JM, Diabetes insipidus: StatPearls, 2018, StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK470458/
19.. Barnabei A, Strigari L, Corsello A, Grading central diabetes insipidus induced by immune checkpoint inhibitors: A challenging task: Front Endocrinol (Lausanne), 2022; 13; 840971
20.. Murdaca G, Russo R, Spanò F, Autoimmune central diabetes insipidus in a patient with ureaplasma urealyticum infection and review on new triggers of immune response: Arch Endocrinol Metab, 2015; 59(6); 554-58
21.. Côté M, Salzman KL, Sorour M, Couldwell WT, Normal dimensions of the posterior pituitary bright spot on magnetic resonance imaging: J Neurosurg, 2014; 120(2); 357-62
22.. Li Y, Shaw S, Kamsteeg E-J, Vandewalle A, Deen PMT, Development of lithium-induced nephrogenic diabetes insipidus is dissociated from adenylyl cyclase activity: J Am Soc Nephrol, 2006; 17(4); 1063-72
23.. Cuzzo B, Padala SA, Lappin SL, Physiology, vasopressin: StatPearls, 2022, StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK526069/
24.. Song Z, Sidorowicz HE, Sladek CD, cAMP stimulation of vasopressin and oxytocin release and regulation of vasopressin mRNA stability: Role of auto-facilitation: J Neuroendocrinol, 2001; 13(2); 158-65
25.. Sugawara M, Hashimoto K, Hattori T, Effects of lithium on the hypothalamo-pituitary-adrenal axis: Endocrinol Jpn, 1988; 35(5); 655-63
26.. Mutter CM, Smith T, Menze O, Diabetes insipidus: Pathogenesis, diagnosis, and clinical management: Cureus, 2021; 13(2); e13523
Figures
Tables
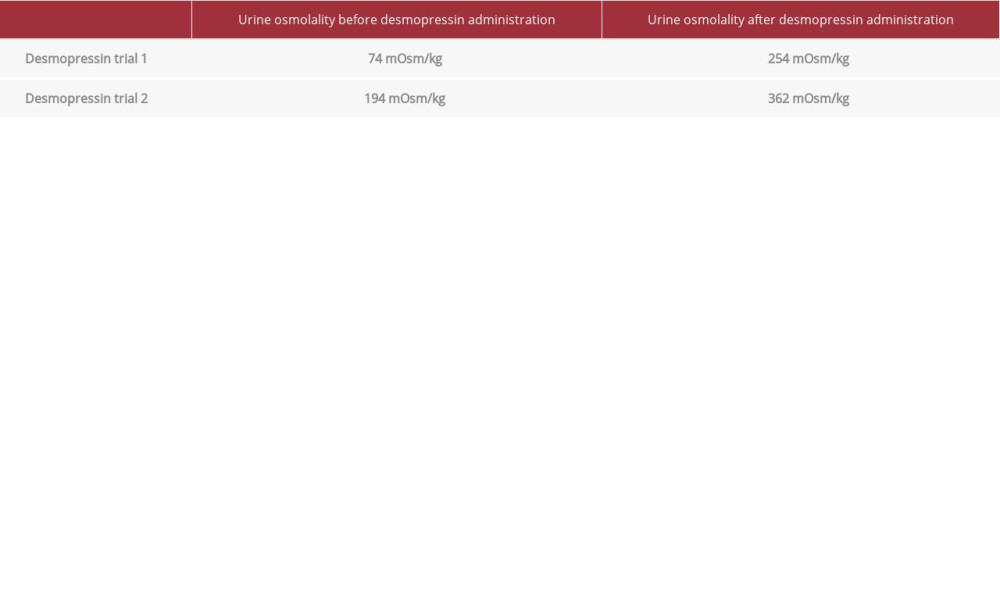 Table 1.. Urine osmolality after desmopressin trials. First desmopressin trial resulted in a 343% increase in urine osmolality. Second desmopressin trial resulted in a 187% increase in urine osmolality. Increase in urine osmolality by at least 50% after desmopressin administration is diagnostic of central diabetes insipidus.
Table 1.. Urine osmolality after desmopressin trials. First desmopressin trial resulted in a 343% increase in urine osmolality. Second desmopressin trial resulted in a 187% increase in urine osmolality. Increase in urine osmolality by at least 50% after desmopressin administration is diagnostic of central diabetes insipidus. Table 1.. Urine osmolality after desmopressin trials. First desmopressin trial resulted in a 343% increase in urine osmolality. Second desmopressin trial resulted in a 187% increase in urine osmolality. Increase in urine osmolality by at least 50% after desmopressin administration is diagnostic of central diabetes insipidus.
Table 1.. Urine osmolality after desmopressin trials. First desmopressin trial resulted in a 343% increase in urine osmolality. Second desmopressin trial resulted in a 187% increase in urine osmolality. Increase in urine osmolality by at least 50% after desmopressin administration is diagnostic of central diabetes insipidus. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








