11 June 2024: Articles 
Remarkable Recovery After Severe Gunshot Brain Injury: A Comprehensive Case Study of Functional Rehabilitation
Unusual clinical course, Management of emergency care
Christos Tsitsipanis12ABDEF, Ioanna Papadimitriou3BDEF, Ioannis Tsoukaras4ABDEF, Nikolaos Moustakis1DEF, Sofia Lazarioti1DEF, Athanasios K. Theofanopoulos1BDF, Georgia Kritikou5EF, Konstantinos Ntotsikas1DEF*, Panagiotis Simos6ADEF, Emmanouil Kokkinakis7BDF, Dimitris KarabetsosDOI: 10.12659/AJCR.941601
Am J Case Rep 2024; 25:e941601
Abstract
BACKGROUND: Penetrating traumatic brain injury (TBI) caused by gunshots is a rare type of TBI that leads to poor outcomes and high mortality rates. Conducting a formal neuropsychological evaluation concerning a patient’s neurologic status during the chronic recovery phase can be challenging. Furthermore, the clinical assessment of survivors of penetrating TBI has not been adequately documented in the available literature. Severe TBI in patients can provide valuable information about the functional significance of the damaged brain regions. This information can help inform our understanding of the brain’s intricate neural network.
CASE REPORT: We present a case of a 29-year-old right-handed man who sustained a left-hemisphere TBI after a gunshot, causing extensive diffuse damage to the left cerebral and cerebellar hemispheres, mainly sparing the right hemisphere. The patient survived. The patient experienced spastic right-sided hemiplegia, facial hemiparesis, left hemiparesis, and right hemianopsia. Additionally, he had severe global aphasia, which caused difficulty comprehending verbal commands and recognizing printed letters or words within his visual field. However, his spontaneous facial expressions indicating emotions were preserved. The patient received a thorough neuropsychological assessment to evaluate his functional progress following a severe TBI and is deemed to have had a favorable outcome.
CONCLUSIONS: Research on cognitive function recovery following loss of the right cerebral hemisphere typically focuses on pediatric populations undergoing elective surgery to treat severe neurological disorders. In this rare instance of a favorable outcome, we assessed the capacity of the fully developed right hemisphere to sustain cognitive and emotional abilities, such as language.
Keywords: case reports, Cerebrum, Head Injuries, Penetrating, Neurobehavioral Manifestations, Wounds, Gunshot
Introduction
Penetrating traumatic brain injury (TBI) is considered a rare form of TBI [1–3]. It is widely acknowledged in the literature that this type of TBI leads to worse outcomes than other forms of TBI [1,4–6]. In addition, head injuries caused by gunshot wounds that affect the brain are less common and often fatal [1,6,7]. Gunshot injuries to the brain can cause significant damage to the parenchyma due to ballistics [1]. Studies indicate that civilians who experience TBI caused by gunshot wounds have a high chance of unfavorable outcomes, with a rate of nearly 97%, including fatalities [1,6,8]. No clear definition exists for favorable or unfavorable outcomes; however, an upper severe disability is often used as the lower cutoff for favorable outcome when the extended Glasgow Outcome Scale is used as the method to measure outcomes [8,9]. Additionally, if a patient can be unsupervised for at least 8 h but is unable to utilize public transportation, they can be considered to have a favorable outcome [9]. Literature data consists mainly of small-sample studies and case reports [1]. Furthermore, most extensive studies originate from military databases focusing on military personnel [1,4]. It is crucial to acknowledge that there could be substantial variations between civilian and military populations when collecting data from military personnel studies [5]. It is important to note that the findings of military research cannot be generalized to other groups and populations [5]. Civilian gunshot penetrating TBIs are usually caused by low-velocity bullets (300 m per second), transmitting lower forces to the brain parenchyma [2,10]. It appears that gunshot penetrating TBI outcomes may be strongly influenced by the admission Glasgow Coma Scale (GCS) score and the number of lobes injured [2,3,7,10,11]. In addition, the trajectory of the bullet within the cranium is considered a significant ballistics characteristic, as it can serve as a prognostic factor [12]. Survival of patients after severe penetrating brain injury is rare [1,6]. Consequently, the prospect of examining a survivor of such injury is a rarity. The examination of patients who have acquired brain lesions has the potential to advance our understanding of the functional roles of the damaged brain regions. Moreover, follow-up examination of such patients could help us estimate the degree of brain’s plasticity capacity in adulthood. In light of the limited data available, the acquisition of novel insights regarding the functional outcomes of patients affected by severe gunshot penetrating TBI ought to be regarded as significant. The dominance of the left hemisphere in relation to language skills is a well-established fact [13,14]. Before the left specialization occurs, Lenneberg’s theory suggests that language skills may be developed in the non-dominant hemisphere [13,14]. Numerous studies in the literature have shown strong support for the development of language skills in patients who have had functional surgical removal of their left hemisphere to treat severe neurological disorders, such as Rasmussen encephalitis or seizures in their youth [13,15]. According to the study conducted by Borne et al, 3 patients who underwent left functional hemispherectomy as a treatment for Rasmussen encephalitis experienced favorable functional outcomes [13]. Moreover, studies have shown that Rasmussen encephalitis and vascular etiology leading to left cerebral hemispherectomy during childhood result in improved outcomes [16]. It is essential to mention that limited information is available about the cognitive recovery of adult patients who have experienced a severe TBI in their left hemisphere. Therefore, we report the case of a 29-year-old right-handed man who experienced near complete loss of the left cerebral hemisphere, caused by a gunshot head wound. This patient afforded us the opportunity to assess elementary cognitive, language, and emotional processing capacities, given that the patient was able to comply with simple verbal commands.
Case Report
A 29-year-old man (a farmer with 6 years of formal education) was transferred to the Emergency Department of our hospital in a comatose state (GCS score 3/15) approximately 1 h after being shot to the head. His pupils were reactive to light and normal-sized. There was an open left temporal wound with active bleeding, which was sutured immediately. After the intubation procedure, an urgent computed tomography (CT) scan was conducted, revealing the point of entry and trajectory of the bullet (Figures 1–4). An exit point could not be identified. The fragments of the bullet, along with the bony fragments, were recognized. Figures 5–7 present a 3-dimensional reconstruction that facilitates a comprehensive understanding of the bullet’s trajectory. Subsequently, he underwent an emergency left decompressive craniectomy, removal of subdural hematoma, and removal of 2 bullet fragments. The removal of the largest fragments was not feasible. After surgery, the patient was transferred to the Intensive Care Unit (ICU) intubated, where an intracranial catheter was placed, revealing elevated intracranial pressure of 40 mmHg. A new CT scan showed extensive brain edema, multiple large hemorrhagic lesions in the left hemisphere, and incipient obstructive hydrocephalus (Figures 8–10). Henceforward, 2 external ventricular drainages were placed in both lateral ventricles. Following reduction of intracranial pressure to 11 mmHg, sedation was withdrawn. Upon neurological examination, he could open his eyes spontaneously and localize to painful stimuli.
The entrance wound was situated posterior to the zygomatic process of the temporal bone. Fragmented bullets or ricochets of the bone can complicate the determination of exit sites [17]. Hence, treating the wounds as they present and avoiding labeling site of bullet entrances is advised due to the high mis-classification rate [17]. Furthermore, it was observed that the trajectory of the bullet did not cross the midline. Instead, it followed a slanted path in close proximity to the medial aspect of the occipital bone on the ipsilateral side. On the CT of the brain, metallic and osseous remnants were detected along its path. Unfortunately, information regarding the ballistics of the gunshot is confined to a low-velocity shot discharged from a handgun at a range of approximately 10 to 15 m.
After 2.5 months, while hospitalized, the patient underwent cranioplasty. The post-cranioplasty CT scan is provided in Figure 11. Furthermore, a gastrostomy procedure was performed to ameliorate the patient’s nutritional status and minimize the risk of aspiration pneumonia, given the patient’s compromised level of consciousness and inability to feed unaided. During his hospitalization, follow-up CT scans were performed. The patient developed spastic right-sided hemiplegia; thus, a lumbar baclofen pump was placed to reduce spasticity. Further improvement of neurological status was noted, as the patient displayed simple motor responses to verbal stimuli that did not imply comprehension.
Upon discharge, he presented with spastic right-sided hemiplegia and facial hemiparesis, left hemiparesis involving the hand and foot, and right hemianopsia. He could not execute voluntary movements of the orofacial muscles, and did not show evidence of comprehending simple verbal commands or questions and recognizing printed letters or words presented within his intact visual field. Therefore, his language ability profile was consistent with severe global aphasia. Spontaneous facial expressions indicating negative or positive emotions were preserved. After discharge from the hospital, he was transferred to an inpatient rehabilitation center, where he remained for 19 months. During the patient’s hospitalization, our department provided daily physiotherapy sessions. Later at the rehabilitation center, the patient received daily physiotherapy, ergotherapy, and weekly logotherapy sessions. On a follow-up visit to our department, 2 years after the incident, his motor status remained unchanged, with slight improvement in oro-facial motility. Language assessment using subtests from the Boston Diagnostic Aphasia Examination revealed severe oro-motor and verbal dyspraxia, and complete loss of the ability to write and recognize letters and words. His verbal comprehension ability was severely limited, even for recognition of the spoken name of highly familiar objects, which is characteristic of global dysphasia. Severely restricted attention span and willingness to cooperate in the assessment and speech therapy were noted, with rapid improvement over the next 6 to 8 months, when he received weekly speech therapy.
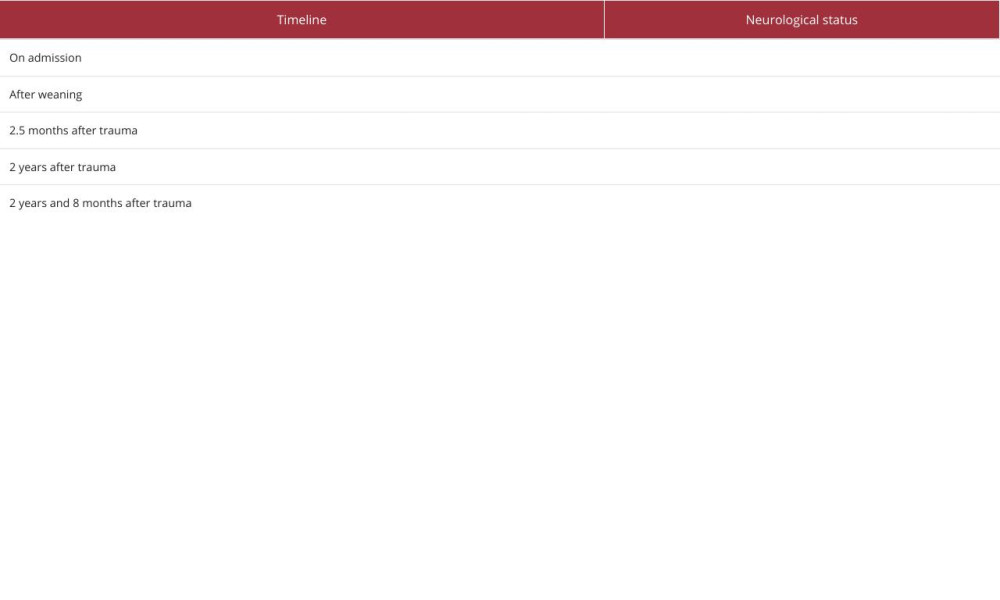
On the next follow-up, 8 months later, spastic right-sided hemiplegia and left-sided hemiparesis persisted. Orofacial hemiparesis was rated as mild, with notable improvement in the capacity to execute largely symmetric lip and tongue movements upon request. Spontaneous speech was restricted to monosyllabic utterances produced in response to task instructions or spontaneously, typically to indicate that he wanted to return home. We did not elicit intelligible naming or repetition responses. The patient could follow up to 2-stage spoken commands, for example, “pick the pencil from the table and give it to the doctor” when he was able to pick the correct 1 of 2 pencils and pass it to the correct of 2 persons seated in front of him. He was able to pick out the correct, orally presented target from 4-object arrays, using his left hand (more readily with real-life objects than pictures). Further, he recognized 1- and 2-digit numbers on the auditory-visual matching task. His facial expressions were preserved, as on the first follow-up visit. Informant report and examiner observations converged with respect to the patient’s capacity to generate emotional expressions (facial and vocal) of joy, sadness (or discontent), and fear in response to video clips, internal triggers (such as hunger and boredom), and external real-life contextual cues (a familiar view at the scenery outside his country home, the sight of a loved one approaching, as well as various spoken statements – for instance that he would have to tolerate additional testing, or that they were ready to leave the hospital). Informant reports indicated extensive preservation of episodic memories acquired before injury and some capacity to learn new non-verbal information (faces, locations, and routes). Table 1 presents a concise overview of the recovery process, outlining the essential steps.
Neurological and basic cognitive assessment was conducted on 2 follow-up visits. In addition to the standard neurological assessment, basic oromotor and language capacities were assessed through tasks adapted from selected sub-scales of the Greek version of the Boston Diagnostic Aphasia Examination [18]. Emotional processing capacity was assessed through examiner ratings of the presence or absence of appropriate or inappropriate facial expressions upon presentation of 3 short video clips. Retrograde and anterograde amnesia was assessed using informant ratings.
Discussion
Gunshot-related TBI presents a significant challenge for medical professionals due to its high mortality rate [1,4–7]. Pre-hospital mortality rates are as high as 90%, while only half of those who arrive alive at the hospital survive [19]. Admission GCS is considered the strongest prognostic factor of survival following penetrating head injuries, along with pupil size/reactivity, bullet trajectory [3,7,8,11], neuroimaging findings on CT [3], number of lobes affected [3,10], lesion location, and demographics [11]. Furthermore, the path of the bullet and its projectiles, as well as the amount of energy applied, are crucial factors in determining the extent of injury [17]. These elements have a significant impact on the outcome [17]. Rothschild and Schneider reported on a unique case involving a 28-year-old patient who survived a gunshot wound to the head despite being left for dead for 2 days [20]. The patient experienced only minor neurological impairment and was able to resume his regular daily routine and job [20]. The authors speculated that the patient’s favorable outcome was due to low-impact energy and the bullet trajectory, which did not include eloquent brain areas [20].
Intracranial pressure greater than 20 mmHg and GCS of 3-4 at presentation were moderately associated with higher mortality [8]. Patients with a GCS >8, normal pupil reaction, and single lobe brain injury, including those with GCS ranging between 8–13 with uncompromised ventricles, can benefit from early and aggressive surgical treatment [3,7]. On the other hand, advancements in medical knowledge and emergency response make it reasonable to assume that previously devastating injuries can be treated, with better outcomes. Interestingly, a study was conducted by Joseph et al to delve into the effects of aggressive resuscitation on patients with gunshot TBI who were admitted to a level I trauma center [19]. The objective of the study was to examine the influence of this approach on patient outcomes and organ donation rates [19]. The findings indicated a steady improvement in survival rates over a 5-year span [19]. In addition, Qi and Li presented a case of a patient with a severe gunshot-induced TBI who demonstrated a favorable outcome [21]. The patient’s admission GCS was 6, with anisocoria and a gunshot wound on the right frontal bone [21]. The projectiles did not cross the midline, and the patient exhibited a favorable outcome after aggressive treatment [21]. However, current medical literature falls short in providing comprehensive guidelines for bullet management [17]. It is worth noting that inappropriate extraction can result in various complications, such as damage to surrounding tissues, infection, neurovascular injury, deep vein thrombosis, and excessive bleeding [17].
The most frequent causes of near complete left hemisphere damage are hemispherectomy, stroke, and trauma [14,22,23]. Nearly all single-case studies and case series reports concern left hemispherectomies to manage medical conditions associated with congenital or early-onset damage or disease, such as epilepsy and/or hemiplegia, Rasmussen encephalitis, and Sturge-Weber syndrome [2,3,8,11]. Most commonly, removal of a severely impaired/malformed hemisphere took place in childhood [24–27]. Significant language preservation and recovery has also been observed following adult hemispherectomies [23,25,28].
In their case report, Wang et al reported an adult patient who was treated with functional left hemispherectomy to treat late-onset Rasmussen encephalitis; however, it should be noted that remodeling of the brain cortex responsible for language skills might have occurred gradually due to chronic development of the disease [29]. Moreover, in one of the studies with a larger population, Liang et al demonstrated improvement in motor and cognitive function in patients with severe unilateral epilepsy that underwent left hemispherectomy [25]. Although, concerning surgical removal of the left hemisphere for treatment of epilepsy, our results are in accordance with the report of Fournier et al about the functional modalities of the right hemisphere and improvement in language skills [30]. Kliemann et al presented evidence in their case series study indicating that cortical reorganization during adulthood may be a potential explanation for the enhancement of socioemotional processing functions [31]. Regarding our results, it is possible to hypothesize that there was a cortical reorganization in the contralateral homologous regions responsible for processing language skills.
Early and aggressive treatment for patients with severe penetrating gunshot TBI seems to be a reasonable approach [19]. According to the findings presented by Kim et al, it is assumed that favorable outcomes can be achieved through optimal surgical intervention [2]. Whereas comprehensive neuropsycho-logical evaluation was possible in most of these reports, cognitive outcomes following complete left hemisphere damage secondary to penetrating TBI are very rare. The patient in the present case displayed remarkable recovery of receptive language functions despite limited speech therapy or other systematic cognitive interventions, accompanied by near-complete resolution of facial paresis and hemiplegia. This progress was noted mainly during the third year after injury, suggesting that brain mechanisms within the partially intact right hemisphere developed gradually, to subsume impaired language functions. Although anterograde amnesia for verbal material could not be assessed in our patient, informant reports suggested partially preserved episodic learning capacity for faces, spatial contexts, and routes. Concerning emotional processing, the patient appeared capable of evaluating dynamic, visual stimuli conveying emotional information for joy, sadness, and fear (video clips) and generating appropriate facial emotional expressions. This is in line with the notion that the isolated right hemisphere is not merely capable of supporting this function but may actually display greater specialization than the left hemisphere for emotion processing, regardless of valence, in other words, for both positive and negative emotions [22].
Culmination regarding the mechanisms responsible for preserving and/or recovering cognitive functions are inevitably limited by lack of brain imaging data assessing right hemisphere hemodynamic/metabolic viability and function. Moreover, functional status evaluation was restricted to tasks assessing mainly receptive language abilities and informant reports of memory capacity.
As an important consideration, we wish to highlight several limitations of our study. First, we would like to express that the available information on the ballistics of the bullet was insufficient, which in turn, impacted the clarity of our findings. Second, we acknowledge that the assumptions we made regarding the functional outcome of this case relied on a limited amount of literature, which makes it difficult to generalize our results and conclusions. Lastly, we acknowledge that confounding factors that may have impacted the patient’s final outcome were difficult to interpret, leading to some uncertainty in our findings.
Conclusions
Surviving a gunshot wound to the head is extremely rare and can lead to challenges for clinicians in conducting a comprehensive neuropsychological evaluation during the chronic phase of recovery. However, we recently had the opportunity to assess a unique case that showed promising results. By examining the functioning of the mature right hemisphere, we were able to gain insights into how the brain can recover and adapt following such a traumatic event. This information can be invaluable in understanding residual cognitive and emotional functions after a severe injury. Furthermore, it should be noted that the patient was admitted to our hospital with a GCS score of 3 and anisocoria. However, early and aggressive treatment achieved a favorable outcome. In addition, the final outcome presented in late fashion. As a result, we consider the notion that aggressive treatment can be justifiable in patients with severe TBI, even if their prognosis is assessed to be poor.
Figures
References:
1.. Deng H, Yue JK, Winkler EA, Adult firearm-related traumatic brain injury in United States trauma centers: J Neurotrauma, 2019; 36(2); 322-37
2.. Kim TW, Lee JK, Moon KS, Penetrating gunshot injuries to the brain: J Trauma, 2007; 62(6); 1446-51
3.. Hofbauer M, Kdolsky R, Figl M, Predictive factors influencing the outcome after gunshot injuries to the head-a retrospective cohort study: J Trauma, 2010; 69(4); 770-75
4.. Dikmen SS, Corrigan JD, Levin HS, Cognitive outcome following traumatic brain injury: J Head Trauma Rehabil, 2009; 24(6); 430-38
5.. DuBose JJ, Barmparas G, Inaba K, Isolated severe traumatic brain injuries sustained during combat operations: Demographics, mortality outcomes, and lessons to be learned from contrasts to civilian counterparts: J Trauma, 2011; 70(1); 11-16 ; discussion 16–18
6.. D’Agostino R, Kursinskis A, Parikh P, Management of penetrating traumatic brain injury: operative versus non-operative intervention: J Surg Res, 2021; 257; 101-6
7.. Martínez-Bustamante D, Pérez-Cárdenas S, Ortiz-Nieto JM, [Craniocerebral gunshot wounds in civilian population: Analysis of experience in a single center in Monterrey, México]: Cir Cir, 2015; 83(2); 94-99 [in Spanish]
8.. Gressot LV, Chamoun RB, Patel AJ, Predictors of outcome in civilians with gunshot wounds to the head upon presentation: J Neurosurg, 2014; 121(3); 645-52
9.. Zuckerman DA, Giacino JT, Bodien YG, Traumatic brain injury: What is a favorable outcome?: J Neurotrauma, 2022; 39(13–14); 1010-12
10.. Khan MB, Kumar R, Irfan FB, Civilian craniocerebral gunshot injuries in a developing country: Presentation, injury characteristics, prognostic indicators, and complications: World Neurosurg, 2014; 82(1–2); 14-19
11.. Aarabi B, Tofighi B, Kufera JA, Predictors of outcome in civilian gunshot wounds to the head: J Neurosurg, 2014; 120(5); 1138-46
12.. Turco L, Cornell DL, Phillips B, Penetrating bihemispheric traumatic brain injury: A collective review of gunshot wounds to the head: World Neurosurg, 2017; 104; 653-59
13.. Borne A, Perrone-Bertolotti M, Jambaqué I, Cognitive outcome after left functional hemispherectomy on dominant hemisphere in patientswith Rasmussen encephalitis: Beyond the myth of aphasia. Patient series: J Neurosurg Case Lessons, 2022; 4(22); CASE22410
14.. Bulteau C, Jambaqué I, Chiron C, Language plasticity after hemispherotomy of the dominant hemisphere in 3 patients: Implication of non-linguistic networks: Epilepsy Behav, 2017; 69; 86-94
15.. Liu D, Guan Y, Zhou J, The influencing factors and changes of cognitive function within 40 Rasmussen encephalitis patients that received a hemispherectomy: Neurol Res, 2022; 44(8); 700-7
16.. Nahum AS, Liégeois FJ, Language after childhood hemispherectomy: A systematic review: Neurology, 2020; 95(23); 1043-56
17.. Baum GR, Baum JT, Hayward D, MacKay BJ, Gunshot wounds: Ballistics, pathology, and treatment recommendations, with a focus on retained bullets: Orthop Res Rev, 2022; 14; 293-317
18.. Papathanassiou E, Papadimitriou D, Gavrilou V, Michou A, Normative data for the Boston Diagnostic Aphasia Battery in Greek: gender and age effects: Psychology: The Journal of the Hellenic Psychological Society, 2020; 15(2); 23845
19.. Joseph B, Aziz H, Pandit V, Improving survival rates after civilian gunshot wounds to the brain: J Am Coll Surg, 2014; 218(1); 58-65
20.. Rothschild MA, Schneider V, Gunshot wound to the head with full recovery: Int J Leg Med, 2000; 113(6); 349-51
21.. Qi H, Li K, Civilian gunshot wounds to the head: A case report, clinical management, and literature review: Chin Neurosurg Jl, 2021; 7(1); 12
22.. Abbott JD, Cumming G, Fidler F, Lindell AK, The perception of positive and negative facial expressions in unilateral brain-damaged patients: A meta-analysis: Laterality, 2013; 18(4); 437-59
23.. Cummings JL, Benson DF, Walsh MJ, Levine HL, Left-to-right transfer of language dominance: A case study: Neurology, 1979; 29(11); 1547-50
24.. Bulteau C, Grosmaitre C, Save-Pédebos J, Language recovery after left hemispherotomy for Rasmussen encephalitis: Epilepsy Behav, 2015; 53; 51-57
25.. Liang S, Zhang G, Li Y, Hemispherectomy in adults patients with severe unilateral epilepsy and hemiplegia: Epilepsy Res, 2013; 106(1–2); 257-63
26.. Mariotti P, Iuvone L, Torrioli MG, Silveri MC, Linguistic and non-linguistic abilities in a patient with early left hemispherectomy: Neuropsychologia, 1998; 36(12); 1303-12
27.. Liégeois F, Connelly A, Baldeweg T, Vargha-Khadem F, Speaking with a single cerebral hemisphere: fMRI language organization after hemispherectomy in childhood: Brain Lang, 2008; 106(3); 195-203
28.. Burklund CW, Smith A, Language and the cerebral hemispheres. Observations of verbal and nonverbal responses during 18 months following left (“dominant”) hemisphrerectomy: Neurology, 1977; 27(7); 627-33
29.. Wang Q, Zhu Z, Wang G, Functional hemispherectomy for adult rasmussen encephalitis: A case report and literature review: Turk Neurosurg, 2019; 29(6); 945-49
30.. Fournier NM, Calverley KL, Wagner JP, Impaired social cognition 30 years after hemispherectomy for intractable epilepsy: The importance of the right hemisphere in complex social functioning: Epilepsy Behav, 2008; 12(3); 460-71
31.. Kliemann D, Adolphs R, Paul LK, Reorganization of the social brain in individuals with only one intact cerebral hemisphere: Brain Sci, 2021; 11(8); 965
Figures
In Press
Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943422
Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.944035
Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.944106
Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.944396
Most Viewed Current Articles
07 Mar 2024 : Case report  38,790
38,790
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report  31,201
31,201
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
23 Feb 2022 : Case report  17,908
17,908
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250
19 Jul 2022 : Case report  17,850
17,850
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128