10 April 2024: Articles 
A Rare Cause of Empyema and Bacteremia Due to Species in Alcoholic Cirrhosis Patients: A Case Report and Comprehensive Review of Literature
Unusual clinical course
Bohui Qian1BCDEF, Kazuhiro Ishikawa1ABDEF*, Tomoaki Nakamura2CDEF, Katsuhito Kinoshita2CDEF, Tetsuhiro Masaki1CF, Takahiro Matsuo1ADE, Fujimi KawaiDOI: 10.12659/AJCR.941952
Am J Case Rep 2024; 25:e941952
Abstract
BACKGROUND: Shewanella spp. are gram-negative facultative anaerobic, oxidase-positive, motile bacilli that are ubiquitous but commonly occur in seawater and can cause opportunistic infection. Reports on the risk factors for Shewanella infection, its severity, antibiotic susceptibility, and prognosis are limited. This report is of a 78-year-old man with alcoholic cirrhosis presenting with bacteremia and empyema due to infection with Shewanella spp.
CASE REPORT: A 78-year-old man with alcoholic cirrhosis (Child-Pugh B) presented to our emergency room with a high fever. He had eaten raw fish one week prior to admission. Chest computed tomography showed a right unilateral pleural effusion, and he was hospitalized with suspected empyema. Shewanella spp. was detected in the pleural effusion and blood cultures. We initiated piperacillin/tazobactam and vancomycin empirically and switched to ceftriaxone; the effusion was successfully treated using antibiotics and pleural drainage. However, on hospitalization day 53, the patient died of aspiration pneumonia. In our literature review, we extracted 125 reported cases (including our case) and found that men were disproportionately affected (81%); median age was 61.6 (56-75) years; underlying diseases included hepatobiliary disease (33%), malignancy (25%), and cardiac disease (24%); Shewanella spp. infection sites were skin and soft tissue (35%), respiratory system (18%), and hepatobiliary system (11%); and management included antibiotics (100%), drainage (16%), and debridement (16%). The survival rate was 74% with antibiotics alone.
CONCLUSIONS: Our case highlights that clinicians should recognize Shewanella spp. as a cause of empyema and bacteremia in patients with liver cirrhosis, and that microbiological diagnosis with antibiotic sensitivity testing and treatment should be undertaken urgently to prevent fatal sepsis.
Keywords: Bacteremia, Empyema, Shewanella, Shewanella algae
Introduction
Case Report
In this study, we report a case of empyema and bacteremia due to
On the day of admission, we inserted a chest tube to drain the empyema on the right side and started piperacillin/tazobactam (4.5 g every 8 h) and vancomycin. Cultures of blood and pleural effusion were positive for
Discussion
We encountered a case of a patient with liver cirrhosis who developed
In general,
We retrieved a total of 330 articles (80 from PubMed, 99 from Embase, and 151 from Ichushi). After removing records not reporting bacteremia due to
The results of the literature review and the case list are presented in Tables 2 and 3 [7–69].
Of the published cases, 35 were from the United States of America, 28 were from South Africa, 16 were from Japan, and 12 were from Taiwan. An analysis of all 124 patients from the studies revealed that 39 (31%) were infected with
In terms of portal of entry, skin lesions, seen in 21/35 patients (60%), constituted the most common portal, while an oral portal of entry was the second most common, seen in 9/35 patients (26%). The consumption of raw fish was assumed to be the cause of infection in the present case. There have been numerous reports of
In this literature review,
In terms of underlying diseases, hepatobiliary disease may be a result of
Some studies have reported that the prognosis of
This study had several limitations. As mentioned above, 16S rRNA gene sequencing analysis is more accurate than MALDITOF-MS in identifying
Conclusions
We report a case of
Figures
Tables
Table 1.. Minimum inhibitory concentrations required for antibiotic efficacy against Shewanella algae.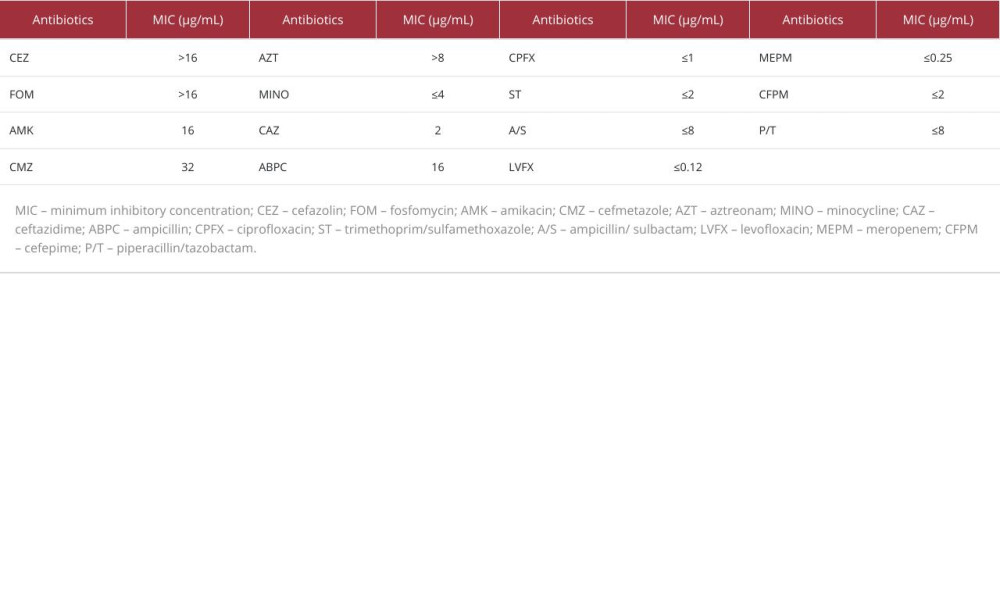 Table 2.. Baseline characteristics of patients, management of infection, and prognosis.
Table 2.. Baseline characteristics of patients, management of infection, and prognosis.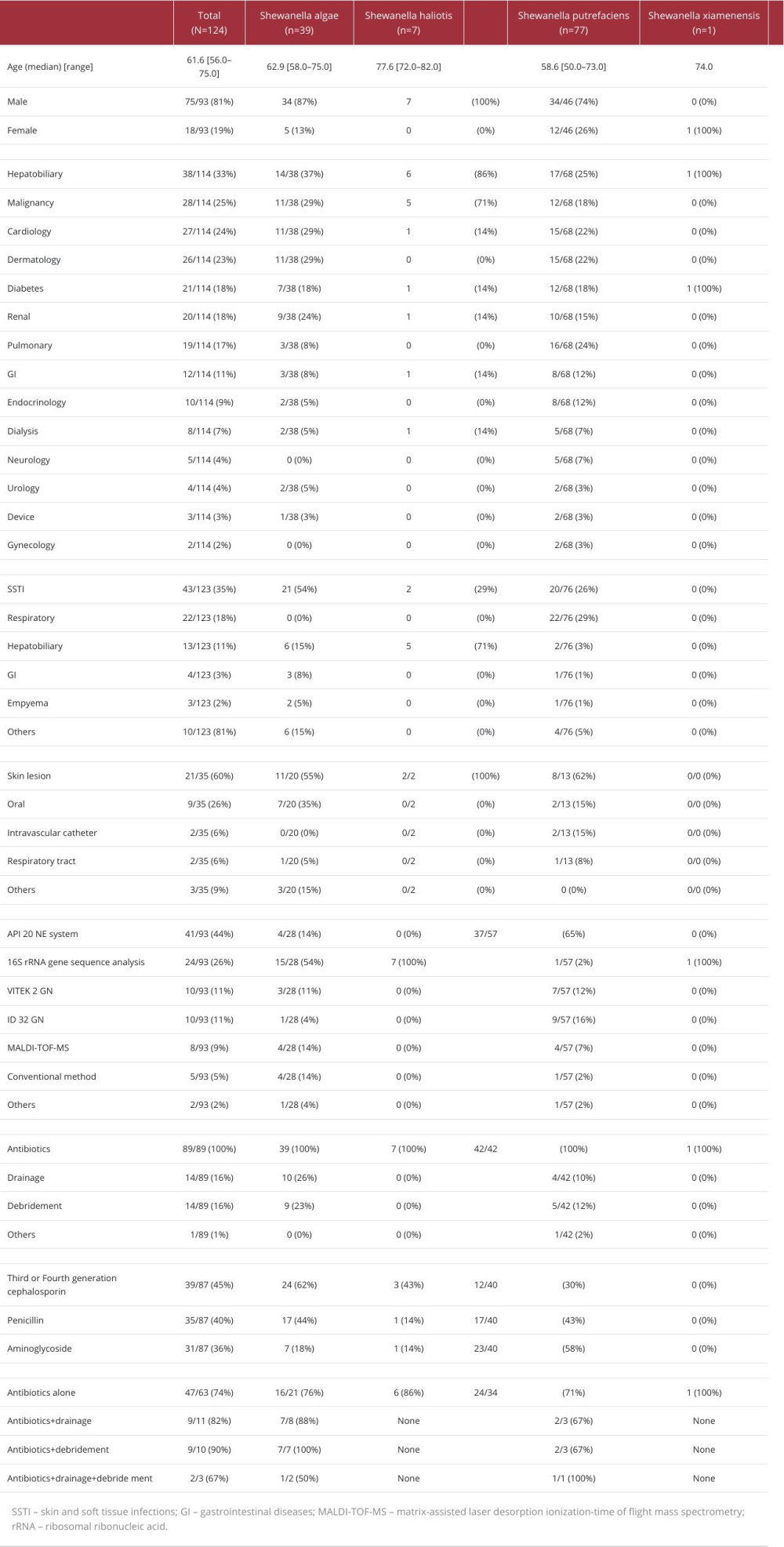 Table 3.. Baseline characteristics, management/treatment, and prognosis of patients with Shewanella spp. bacteremia.
Table 3.. Baseline characteristics, management/treatment, and prognosis of patients with Shewanella spp. bacteremia.
References:
1.. Holt HM, Gahrn-Hansen B, Bruun B: Clin Microbiol Infect, 2005; 11(5); 347-52
2.. Shewan JM, Hobbs G, Hodgkiss W, A determinative scheme for the identification of certain genera of Gram negative bacteria, with special reference to the Pseudomonadaceae: J Appl Bacteriol, 1960; 23(3); 379-90
3.. Ignak S, Unay Demirel O, Soydan S, Esen E: Drug Discov Ther, 2018; 12(2); 108-10
4.. Bravenec CA, Pandit RT, Beaver HA: Indian J Ophthalmol, 2019; 67(1); 148-50
5.. Srinivas J, Pillai M, Vinod V, Dinesh RK: J Clin Diagn Res, 2015; 9(2); DC16-20
6.. Vignier N, Barreau M, Olive C: Am J Trop Med Hyg, 2013; 89(1); 151-56
7.. Jacob-Kokura S, Chan CY, Kaplan L: Ann Pharmacother, 2014; 48(1); 128-36
8.. Yusuke K, Satodate H, Yuki A: J Jpn Soc Surg Inf, 2020; 17(1); 46-53
9.. Kitaoka M, Ishida M, Sakaeda H, [Shewanella algae bacteremia in patients with biliary tract malignancy: Report of three cases.]: Nihon Shokakibyo Gakkai Zasshi, 2019; 116(10); 850-57 [in Japanese]
10.. Kimura H, Mizutomi K, Ota E: J Jpn Assoc Inf Dis, 2018; 92(3); 380-85
11.. Takata T, Chikumi H, Morishita S: Intern Med, 2017; 56(6); 729-32
12.. Roger SD, Chen SC, Lawrence S, Sorrell TC: Nephrol Dial Transplant, 1991; 6(1); 73
13.. Iwata M, Tateda K, Matsumoto T: J Clin Microbiol, 1999; 37(6); 2104-5
14.. Bhandari S, Pan TL, Horvath J, Tiller D: Nephrol Dial Transplant, 2000; 15(9); 1484-85
15.. Jammula P, Gupta R, Agraharkar M: Saudi J Kidney Dis Transpl, 2003; 14(4); 511-15
16.. Yim SY, Kang YS, Cha DR: Perit Dial Int, 2010; 30(6); 667-69
17.. Shrishrimal K: Hemodial Int, 2012; 16(1); 113-15
18.. Lee WS, Ou TY, Chen FL: J Microbiol Immunol Infect, 2016; 49(1); 159-60
19.. Masaki K, Yukie K, Junri C, Takashi S: J Jpn Soc Clin Microbiol, 2017; 27(3); 183-87
20.. Shuto K, Satoshi N, Yuichiro E: HifukanorinshM Rinsho Derma, 2016; 58(12); 1796-97 [in Japanese]
21.. Kanmura M, Kataoka Y, Shinkai T, Takahashi D: J Fujisawa Physicians Assoc, 2013; 25; 19-20 [in Japanese]
22.. Liu P-Y, Lin C-F, Tung K-C: Internal Med, 2013; 52(4); 431-38
23.. Fukunaga M, Takahashi S, Tanimatsu S, Nishiyama M: Ehime J Med Technol, 2013; 32; 77-80 [in Japanese]
24.. Takichi M, Hironori Y, Yoshiko O: Jpn J Clin Dermatol, 2012; 66(9); 662-65
25.. Kayoko T, Akiko S, Kiyofumi O: J Jpn Soc Clin Microbiol, 2010; 20(4); 239-44
26.. Shimizu T, Matsumura Y: Kansenshogaku Zasshi, 2009; 83(5); 553-56 [in Japanese]
27.. Otsuka T, Noda T, Noguchi A, Shewanella infection in decompensated liver disease: A septic case: J Gastroenterol, 2007; 42(1); 87-90
28.. Bernshteyn M, Ashok Kumar P, Joshi S: Cureus, 2020; 12(9); e10676
29.. Hussain A, Gondal M, Yousuf H: Cureus, 2020; 12(8); e9719
30.. Bridwell RE, Carius BM, Oliver JJ: J Spec Oper Med, 2019; 19(4); 19-21
31.. Talbot Z, Amble A, Delva G: Cureus, 2019; 11(9); e5668
32.. Bauer MJ, Stone-Garza KK, Croom D: Open Forum Infect Dis, 2019; 6(11); ofz442
33.. Latif A, Kapoor V, Vivekanandan R, Reddy JT: BMJ Case Rep, 2019; 12(9); e230252
34.. Brugnaro P, Morelli E, Ebo F: Infez Med, 2019; 27(2); 179-82
35.. Raja M, Gonzales Zamora JA, Roig I: IDCases, 2018; 12; 140-41
36.. Ullah S, Mehmood H, Pervin N: J Investig Med High Impact Case Rep, 2018; 6; 2324709618775441
37.. Martin-Rodriguez AJ, Martin-Pujol O, Artiles-Campelo F: JMM Case Rep, 2017; 4(12); e005131
38.. Ranjan R, Chowdhary P: Indian J Pathol Microbiol, 2017; 60(4); 599-600
39.. Davidson NL, Subedi S, Wilks K, Morgan J: BMJ Case Rep, 2018; 2018; bcr2017223396
40.. Giroux PA, Sinna R, Mercut R: Med Mal Infect, 2017; 47(6); 436-38
41.. Brulliard C, Traversier N, Allyn J: Am J Trop Med Hyg, 2017; 97(4); 1043-44
42.. Tang TH, Cheng NH, Ho RT: Open Forum Infect Dis, 2016; 3(3); ofw148
43.. Rajchgot J, Glicksman R, Bogoch II: J Travel Med, 2016; 23(3); taw014
44.. Kim BK, Cho SY, Kang B: Infect Chemother, 2014; 46(4); 264-68
45.. Constant J, Chernev I, Gomez E: Braz J Infect Dis, 2014; 18(6); 686-88
46.. Ananth AL, Nassiri N, Pamoukian VN: Surg Infect (Larchmt), 2014; 15(3); 336-38
47.. Yiallouros P, Mavri A, Attilakos A: Paediatr Int Child Health, 2013; 33(3); 193-95
48.. Garcia-Fragoso L, Garcia-Garcia I, Rivera A: Pediatr Infect Dis J, 2012; 31(1); 104-5
49.. Myung DS, Jung YS, Kang SJ: J Korean Med Sci, 2009; 24(6); 1192-94
50.. Tsai MS, You HL, Tang YF, Liu JW: Int J Infect Dis, 2008; 12(6); e119-24
51.. Vandepitte J, Debois J: J Clin Microbiol, 1978; 7(1); 70-72
52.. Schmidt U, Kapila R, Kaminski Z, Louria D: J Clin Microbiol, 1979; 10(3); 385-87
53.. Eschete ML, Williams F, West BC: Arch Intern Med, 1980; 140(11); 1533-34
54.. Kim JH, Cooper RA, Welty-Wolf KE, Pseudomonas putrefaciens bacteremia: Rev Infect Dis, 1989; 11(1); 97-104
55.. von Graevenitz A, [Pseudobacteremia (author’s transl).]: Schweiz Rundsch Med Prax, 1979; 68(29); 933-37 [in German]
56.. Shimada K, Noro T, Inamatsu T, Bacteriology of acute obstructive suppurative cholangitis of the aged: J Clin Microbiol, 1981; 14(5); 522-26
57.. Heller HM, Tortora G, Burger H, Pseudomonas putrefaciens bacteremia associated with shellfish contact: Am J Med, 1990; 88(1); 85-86
58.. Brink AJ, van Straten A, van Rensburg AJ: Clin Infect Dis, 1995; 20(5); 1327-32
59.. Dominguez H, Vogel BF, Gram L: Clin Infect Dis, 1996; 22(6); 1036-39
60.. Chen YS, Liu YC, Yen MY: Clin Infect Dis, 1997; 25(2); 225-29
61.. Krsnik I, Arribalzaga K, Romanyk J: Haematologia (Budap), 2002; 32(1); 79-80
62.. Pagani L, Lang A, Vedovelli C: J Clin Microbiol, 2003; 41(5); 2240-41
63.. Saidel-Odes L, Borer A, Riesenberg K, Schlaeffer F: Scand J Infect Dis, 2007; 39(4); 360-61
64.. Kim DM, Kang CI, Lee CS: J Clin Microbiol, 2006; 44(3); 1172-74
65.. Wang IK, Lee MH, Chen YM, Huang CC: Chang Gung Med J, 2004; 27(9); 701-5
66.. Paccalin M, Grollier G, le Moal G: Scand J Infect Dis, 2001; 33(10); 774-75
67.. Idowu A, Yau A, Bettoli G, Maritato M: Chest, 2020; 158(4); A840
68.. Solhjoo M, Malik U, Yasmin T: J Investigative Med, 2019; 67(4); 803-5
69.. Blazo J, Plazarte M, Sweet J: J Hosp Med, 2012; 7; S323
70.. Gandiga PC, Gandiga PC: J Gen Intern Med, 2011; 26; S523
71.. Han Z, Sun J, Lv A: Aquaculture, 2017; 468; 356-62
72.. Yan Y, Chai X, Chen Y, Zhang X: Infect Drug Resist, 2022; 15; 1645-50
73.. Ng WW, Shum HP, To KK, Sridhar S: Front Med (Lausanne), 2022; 9; 850938
74.. Byun JH, Park H, Kim S: Jpn J Infect Dis, 2017; 70(2); 177-80
75.. Johnson DH, Cunha BA, Infections in cirrhosis: Infect Dis Clin North Am, 2001; 15(2); 363-71
76.. Yu K, Huang Z, Xiao Y, Wang D: Virulence, 2022; 13(1); 1515-32
77.. Kan J, Flood B, McCrow JP: J Microbiol Methods, 2011; 86(1); 62-68
Figures
Tables
 Table 1.. Minimum inhibitory concentrations required for antibiotic efficacy against Shewanella algae.
Table 1.. Minimum inhibitory concentrations required for antibiotic efficacy against Shewanella algae. Table 2.. Baseline characteristics of patients, management of infection, and prognosis.
Table 2.. Baseline characteristics of patients, management of infection, and prognosis. Table 3.. Baseline characteristics, management/treatment, and prognosis of patients with Shewanella spp. bacteremia.
Table 3.. Baseline characteristics, management/treatment, and prognosis of patients with Shewanella spp. bacteremia. Table 1.. Minimum inhibitory concentrations required for antibiotic efficacy against Shewanella algae.
Table 1.. Minimum inhibitory concentrations required for antibiotic efficacy against Shewanella algae. Table 2.. Baseline characteristics of patients, management of infection, and prognosis.
Table 2.. Baseline characteristics of patients, management of infection, and prognosis. Table 3.. Baseline characteristics, management/treatment, and prognosis of patients with Shewanella spp. bacteremia.
Table 3.. Baseline characteristics, management/treatment, and prognosis of patients with Shewanella spp. bacteremia. In Press
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








