28 June 2023: Articles 
Preoperative Clinical Prediction of Parathyroid Carcinoma: Two Rare Case Presentations and a Review of Literature
Mistake in diagnosis, Rare disease
Sara A. Assiri1AEF*, Dhuha A. Alhumaidi2BF, Sarah A. Alkashgry2CF, Arif Khurshid2AD, Eidha Fawzan Aljuaid3AE, Hussain A. Alharbi4AFDOI: 10.12659/AJCR.940611
Am J Case Rep 2023; 24:e940611
Abstract
BACKGROUND: Parathyroid carcinoma represents about 0.005% of all malignancies and accounts for less than 1% of primary hyperparathyroidism cases. Precise preoperative diagnosis of parathyroid carcinoma is challenging, and it is usually diagnosed postoperatively by histological examination. Early suspicion of parathyroid carcinoma can lead to a more extensive surgical approach to reduce the risk of carcinoma recurrence.
CASE REPORT: The first case involves a 58-year-old woman who presented with severe back pain. An incidental finding on cervical magnetic resonance imaging of a soft-tissue-density mass at the right para-tracheal zone. The large size and the noticeable mass effect pushing the trachea and esophagus to the left side suggested the need for further investigations to rule out malignancy. Initially, it was thought to be a thyroid nodule investigated by fine-needle aspiration that revealed follicular thyroid cancer. After a histopathological examination, it was determined to be a parathyroid carcinoma. The second case involved a 30-year-old woman with a lower-limb tingling sensation. The significantly enlarged mass seen during thyroid ultrasound warranted surgical excision and histopathological analysis to rule out malignancy. Excision of what was considered a parathyroid adenoma revealed a histopathological finding of carcinoma, prompting a hemithyroidectomy. Both patients had high calcium and parathyroid hormone levels preoperatively.
CONCLUSIONS: Preoperative high calcium, intact parathyroid hormone, creatinine, and alkaline phosphatase, in addition to the lymphocyte-to-monocyte ratio and tumor diameter, are suggested to be predictive of parathyroid carcinoma diagnosis and should be carefully analyzed in all patients presenting with primary hyperparathyroidism.
Keywords: Hypercalcemia, Hyperparathyroidism 1, Parathyroid Cancer, Adult, Parathyroid Glands, Female, Humans, Middle Aged, Adult, Parathyroid Neoplasms, Hyperparathyroidism, Primary, Calcium, Neoplasm Recurrence, Local, Parathyroid Hormone, Carcinoma
Background
Parathyroid carcinoma (PC) represents about 0.005% of all malignancies [1] and accounts for less than 1% of primary hyperparathyroidism cases [2]. However, there is international variation in PC’s incidence. It has been reported to be more frequent in Japan, representing 5% of primary hyperparathyroidism cases [3,4], with a slightly higher rate of 5.2% in Italy [5]. This infrequent tumor usually occurs during the fifth decade of life encountered during laboratory investigation of clinical hyperparathyroidism in patients presenting with digestive, urinary, or skeletal concerns [6–10].
Differentiating PC from benign primary hyperparathyroidism is challenging, and no preoperative markers are available [11]. Although serum calcium levels higher than 14 mg/dL associated with parathyroid hormone 10 times higher than the high normal levels can raise the suspicion of malignancy [12], hypercalcemia can be caused by primary hyperparathyroidism due to autonomous gland overactive secretion of parathyroid hormone (PTH) that can be 3–4 times higher, which is most likely to occur in women in their 5th to mid-7th decades of life [13,14]. Therefore, precise preoperative diagnosis of PC is difficult, and it is mostly postoperatively diagnosed by histological examination [15].
Although the main indicators of malignancy are exaggerated hypercalcemia and severely raised serum PTH levels in addition to increased parathyroid gland size [7], this is not the common clinical presentation of PC [11], as non-functioning PC can be encountered in histological examination [16]. A nonfunctioning PC can be misdiagnosed as a thyroid nodule, and single or multiple independently functioning thyroid nodules have been reported at a rate of 8/million in the general population and even higher rates in areas of relative iodine deficiency, and after the age of 50 are more common in males than females [16].
Chemotherapeutic and radiotherapeutic treatments for PC have not been validated [17–19]. Therefore, the criterion standard for PC treatment is entire resection of the thyroid and parathyroid glands (en-bloc resection), provided that a negative margin exists under microscopic examination [17]. Nationwide data describing the overall clinical profiles of PC patients in Saudi Arabia are still scarce. Herein, we present 2 very rare presentations of PC, aiming to describe the clinical presentation, management, and outcome of PC over 5 years from a single Saudi Arabian district. The vague presentations led to the initial misdiagnoses, which makes the currently presented rare cases unique and separate them from previously reported parathyroid carcinoma cases. This work has been reported in line with the SCARE 2020 criteria [20].
Case Reports
FIRST CASE:
A 58-year-old female patient, known to have asthma and hypertension controlled with medications, presented to a family medicine clinic reporting severe back pain for over 2 years, with no history of renal stones or gastrointestinal complaints. Her general physical examination was normal, and examination of her back did not reveal any tenderness on palpation. She had a normal gait and a full range of passive and active motion, intact sensation, and deep tendon reflexes. Her neck examination did not reveal any palpable cervical lymph nodes or masses.
She underwent a lumbar and cervical spine X-ray, which revealed osteopenia and mild spondylotic changes affecting L2 to L3. A spinal MRI of the cervical spine was subsequently performed, which revealed an incidental finding of a soft-tissue-density mass lesion at the right para-tracheal zone of the lower neck, extending to the upper part of the superior mediastinum, measuring 3.5×4.1×4.8 cm with tiny calcifications inside. No significant findings were observed in the lumbar area. The lesion appeared to push the trachea and esophagus to the left side and contacted the lower border of the right thyroid lobe with no separable plane in between.
Further investigations were warranted to determine its nature. Following these findings, a thyroid ultrasound was performed, which revealed a heterogeneous echo pattern of the right thyroid lobe, showing a large inferior nodule measuring 4.3×2.9 cm with solid and cystic lesions within. Additionally, this patient had multiple enlarged cervical lymph nodes, with the largest on the right side measuring 8.8×2 mm and on the left side measuring 6×2 mm, with a loss of fatty hilum. Subsequently, she underwent fine-needle aspiration of what was thought to be a right thyroid lobular nodule, which showed follicular neoplasm.
The patient underwent a hemithyroidectomy of the right side on May 17, 2016. The right thyroid lobe was excised, and a parathyroid mass measuring 4.0×3.5×2.7 cm was identified. The right thyroid lobule weighed 33 g and measured approximately 4×2×1 cm. An attached enlarged nodular structure measuring 4×3.5×2.7 cm was observed, attached to the thyroid by fibromembranous and fibroadipose tissue. One lymph node measuring 0.6 cm was identified. Final histopathological analysis revealed PC (Figure 1) with a free resection margin but focally very close to the tumor cell (less than 0.1 cm). The tumor was focally adherent to the thyroid capsule without thyroid invasion. A level-6 neck lymph node excisional biopsy was performed, and 5 lymph nodes were identified, with no metastatic tumor seen. Our patient began having back pain 2 years before her final diagnosis was reached; she missed a couple of her appointments and hence there was a delay in the proper diagnosis and final management.
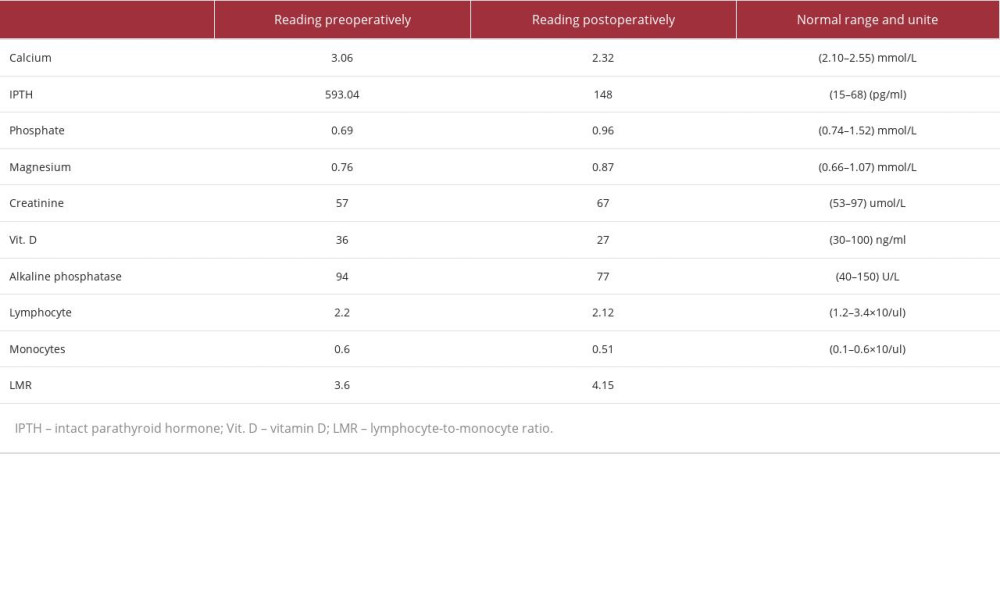
The patient was followed in the clinic for 1 year, with unremarkable laboratory PTH and calcium values and 2 thyroid ultrasound CT scans of the thyroid gland. The patient is still alive and doing well, with no evidence of recurrence. She is following up in the family medicine clinic to record any new symptoms. All her laboratory readings (pre- and post-operative) are demonstrated in Table 1. The decision on the follow-up period was based on clinical judgment, as not protocol has yet been established.
SECOND CASE:
A 30-year-old female patient, medically free, presented to the emergency department in early 2017 with a lower-limb tingling sensation and leg swelling. Deep venous thrombosis was suspected but ruled out.
Similar to the first case, her physical examination was unremarkable. There was no palpable cervical masses or lymph nodes and no bone pain, and lower-limbs examination did not show any tenderness on palpation, with normal lower-limb general appearance and examination.
Initial laboratory investigations revealed hypercalcemia and iPTH of 186 pg/mL. A thyroid ultrasound showed average-sized thyroid lobules and isthmus with no definite solid or cystic lesions and average vascularity. Additionally, multiple enlarged cervical lymph nodes were observed on both sides, with the largest on the right measuring 3.3 × 0.8 cm and the largest on the left measuring 3×0.4 cm, with preserved hilum (this mass was initially reported as a lymph node, and intraoperatively it was identified as a parathyroid enlarged gland).
On January 17, 2017, she underwent resection of a lobulated, red-brown nodular structure measuring 2.5×1.8×1.1 cm. Intraoperative frozen section did not show any vascular or capsular invasion. Final histopathology revealed a left upper PC with capsular and vascular invasion and a margin locally involved (Figure 2). Two weeks following her operation, the clinical decision to re-operate and perform en-block resection (ipsilateral hemithyroidectomy) was made based on the patients’ young age, and no central neck dissection was done as no preoperative radiological investigations revealed any suspicious lymph nodes.
She underwent a left hemithyroidectomy. The left thyroid lobe measured approximately 3.5×2×1 cm and showed unremarkable thyroid tissue with parathyroid foreign body granulomas and with no malignancy observed.
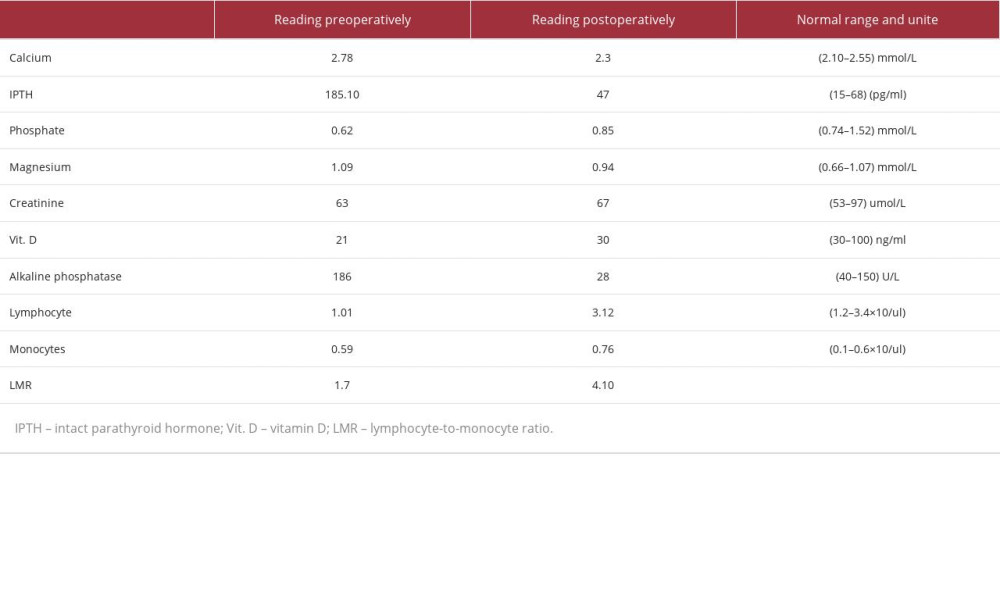
Follow-up with thyroid ultrasound postoperatively showed bilaterally enlarged lymph nodes without any worrisome features and a right thyroid lobe measuring 4.1×1 cm with a homogenous echo pattern. The patient is still alive 5 years 5 months after tumor resection, and is being monitored using serial thyroid ultrasound, laboratory calcium, and iPTH. All her laboratory readings (pre- and post-operative) are demonstrated in Table 2.
Discussion
PC is considered one of the rarest endocrine tumors, affecting less than 1% of individuals with primary hyperparathyroidism. It has significant morbidity and mortality due to its high rate of recurrence and severe hypercalcemia, which can affect multiple organs [21,22]. Early suspicion of PC can lead to a more extensive surgical approach to reduce the risk of recurrence [23]. However, diagnosing PC is challenging because no reliable pre- or intraoperative tools or tests can confirm its presence. The only way to confirm the diagnosis is postoperatively, after histopathological confirmation [24].
Wether undergoing local resection alone, en-bloc resection (including ipsilateral hemithyroidectomy), or radical resection (includes central or lateral neck dissection), there is no debate that surgery is a crucial step in the management of parathyroid cancer [25]. The extent of the surgical approach and avoiding re-operation can be achieved if the diagnosis of parathyroid cancer was made or suspected before or during surgery.
The limited utility of ultrasound-guided fine-needle aspiration (FNA) in diagnosing parathyroid lesions preoperatively is shown by the expected complications such as seeding [26,27], mass disruption [28], and the overlap with thyroid tissue, leading to misdiagnosis [29]. The exact error rate of ultrasound fine-needle aspiration in parathyroid carcinoma has not been properly investigated in the literature, which could be attributed to the rarity of the diagnosis.
Fine-needle aspiration misdiagnosed the first case with thyroid follicular cancer, hence directing the surgical approach towards a single-stage radical surgery involving hemithyroidectomy with mass resection and regional lymph node dissection. Similarly, parathyroid carcinoma has been misdiagnosed previously as thyroid cancer by the fine-needle aspiration results [30,31].
The usual goal of parathyroid imaging is not reaching the diagnosis, but rather to accurately localize the parathyroid tumor. Commonly used imaging modalities include neck ultrasound, 4-dimensional computed tomography (4DCT); and 99m Tcsestamibi scintigraphy scan (MIBI) [32]. Certain signs on thyroid ultrasound such as soft-tissue invasion or parathyroid size of >3 cm [33], lobulation, inhomogeneity, and hypoechogenicity [34] with clinical suspicion can suggest diagnosis of malignancy. A study from the United States found that a slower washout rate on 4DCT scan was noticed in PC patients when compared with benign parathyroid or thyroid tissue, and they concluded that no single imaging modality has enough sensitivity to be used on its own, and optimal localization can be achieved by combining the 3 modalities (US, 4DCT, and MIBI) [35].
Preoperative MIBI scan helps to localize the tumor but it has limited use in distinguishing between benign and malignant parathyroid tissue; however, the degree of delayed uptake has been suggested to be of possible aid in the preoperative diagnosis of PC [36]. MIBI scan has been reported to miss the detection of a metastatic focus in a previously known case of PC. In cases where a sestamibi scan cannot localize a metastatic focus in a patient with known PC, fine-needle aspiration with parathyroid washout is an important secondary study [21].
The second case was initially presumed to be an enlarged cervical lymph node based on the ultrasound result, but the parathyroid hormone and calcium high levels suggested the diagnosis of parathyroid adenoma, which was supported by the initial frozen section done intraoperatively that did not reveal any capsular or vascular invasion. Following the final histopathological diagnosis of parathyroid carcinoma, she underwent left hemithyroidectomy. We do think that the second case could have undergone a single-stage en-block operation if the frozen section came back with clear histopathological signs of malignancy. Intraoperative frozen section can assess the diagnosis; however, its findings can overlap with benign parathyroid disease [37]. A recently published study discussed a promising tool that could be used intraoperatively. The study examined 3 patients using near-infrared (NIR) autofluorescence as a possible tool to identify cancerous tissue. The authors found that the absence of NIR autofluorescence could increase the suspicion of PC [38].
High calcium and intact PTH (iPTH) levels before surgery can raise suspicion of PC, but no reliable cutoff values have been established. However, a calcium level greater than 14 mg/dL may raise suspicion. Patients with PC often have higher creatinine and alkaline phosphatase levels than those with benign parathyroid lesions [39,40]. Interestingly, a study in Korea suggested that an alkaline phosphatase (ALK) value of less than 300 IU/L is less likely to indicate PC, which favors ALK over iPTH as a predictive preoperative factor [41].
Our 2 local patients had an alkaline phosphatase value of less than 300 IU/L (94 and 186 IU/L), which contradicts the results of the aforementioned Korean study. Another study found that a lymphocyte-to-monocyte ratio of less than 4.85 and a long tumor diameter of more than 28 mm had a higher predictive power for PC [42].
This proposed predictive marker was accurate in both of our local patients. The first case had a lymphocyte-to-monocyte ratio of 3.6 and a tumor diameter of more than 28 mm, and the second had a ratio of 1.7 and a tumor diameter of more than 28 mm.
A low T state and N0 stage during the diagnostic period and remission of post-operative biochemical markers have been associated with recurrence-free survival [23]. Both patients presented here had a low T state and no lymph node metastasis. They remained recurrence-free and were in good general condition 5 years after the initial resection.
A large-database study of over 700 PC patients found that those with N1 status did not have a higher risk of death. However, larger tumor size was associated with a higher risk of death for each centimeter increase [22]. The American Joint Committee on Cancer published its first recommendations for a staging system for patients with PC [43]. A group of Chinese scientists found that thyroid invasion (T1 and T2 staging) did not worsen prognosis in patients without lymph nodes or distant metastasis. They also highlighted that once the tumor reached a size of over 4 cm, it was associated with worse cancer-specific survival, regardless of thyroid invasion [44].
There are some anecdotal reports on the long-term remission achieved by using a combination of surgery, chemotherapy, and radiotherapy [45–47]. However, no standard protocols of adjuvant treatment such as chemotherapy or radiotherapy have been developed, as no satisfactory results on the overall survival or recurrence rate have been published [48].
Figure 3 contains all reported cases of PC from different regions in the Kingdom of Saudi Arabia. However, these studies do not necessarily reflect the real incidence of PC in the Saudi population, as no nationwide study has been conducted to assess the incidence of PC. The limited number of reported cases may be attributed to the rarity of this tumor among the Saudi people, which may have a genetic basis. The value of the previously reported cases listed in this figure is unclear due to the low number of cases reported in each study, the lack of clinical, radiological, or intraoperative details, and the absence of long-term follow-up data for most cases presented.
Conclusions
We presented 2 patients with a very rare and vague presentations of PC, along with initial misdiagnoses. PC is considered one of the rarest tumors, and early suspicion and diagnosis are of paramount importance, as it favors a more extended surgical approach to reduce the risk of recurrence. To date, no preor intraoperative tools or tests are reliable for confirming the presence of PC. Preoperative high calcium and iPTH levels can raise suspicion, but no reliable cutoff values have been established. Higher creatinine and alkaline phosphatase levels have been suggested to predict PC diagnosis; however, many studies contradict this, including our 2 reported cases. Another proposed diagnostic parameter is a lymphocyte-to-monocyte ratio of less than 4.85, combined with a tumor diameter over 28 mm. This proposed predictive marker was accurate in both of our local patients. Additionally, our patients had a low T state and no lymph node metastasis and remained cancer-free and in good general condition 5 years after the initial resection.
A nationwide study is warranted to determine the incidence and clinical profiles of PC patients.
Figures
References:
1.. Al-Kurd A, Mekel M, Mazeh H, Parathyroid carcinoma: Surg Oncol, 2014; 23(2); 107-14
2.. Marcocci C, Cetani F, Rubin MR, Parathyroid carcinoma: J Bone Miner Res, 2008; 23(12); 1869-80
3.. Obara T, Okamoto T, Ito Y, Surgical and medical management of patients with pulmonary metastasis from parathyroid carcinoma: Surgery, 1993; 114(6); 1040-48 discussion 1048–49
4.. Obara T, Okamoto T, Kanbe M, Iihara M, Functioning parathyroid carcinoma: Clinicopathologic features and rational treatment: Semin Surg Oncol, 1997; 13(2); 134-41
5.. Favia G, Lumachi F, Polistina F, D’Amico DF, Parathyroid carcinoma: Sixteen new cases and suggestions for correct management: World J Surg, 1998; 22(12); 1225-30
6.. Givi B, Shah JP, Parathyroid carcinoma: Clin Oncol (R Coll Radiol), 2010; 22(6); 498-507
7.. Shane E, Clinical review 122: Parathyroid carcinoma: J Clin Endocrinol Metab, 2001; 86(2); 485-93
8.. Levin KE, Galante M, Clark OH, Parathyroid carcinoma versus parathyroid adenoma in patients with profound hypercalcemia: Surgery, 1987; 101(6); 649-60
9.. Owen RP, Silver CE, Pellitteri PK, Parathyroid carcinoma: A review: Head Neck, 2011; 33(3); 429-36
10.. Kebebew E, Parathyroid carcinoma: Curr Treat Options Oncol, 2001; 2(4); 347-54
11.. Cetani F, Pardi E, Marcocci C, Update on parathyroid carcinoma: J Endocrinol Invest, 2016; 39(6); 595-606
12.. Fingeret AL, Contemporary evaluation and management of parathyroid carcinoma: JCO Oncol Pract, 2021; 17(1); 17-21
13.. Quaglino F, Marchese V, Lemini R, Parathyroid carcinoma. A single Institution experience and a review of the international literature.: Ann Ital Chir, 2018; 89; 295-304
14.. Chen Z, Fu J, Shao Q, 99mTc-MIBI single photon emission computed tomography/computed tomography for the incidental detection of rare parathyroid carcinoma: Medicine (Baltimore), 2018; 97(40); e12578
15.. Cetani F, Pardi E, Marcocci C, Parathyroid carcinoma: Front Horm Res, 2019; 51; 63-76
16.. Vicchio TM, Giovinazzo S, Certo R, Lack of association between autonomously functioning thyroid nodules and germline polymorphisms of the thyrotropin receptor and Gas genes in a mild to moderate iodine-deficient Caucasian population: J Endocrinol Invest, 2014; 37(7); 625-30
17.. Machado NN, Wilhelm SM, Parathyroid cancer: A review: Cancers (Basel), 2019; 11(11); 1676
18.. Medas F, Erdas E, Loi G, Controversies in the management of parathyroid carcinoma: A case series and review of the literature: Int J Surg, 2016; 28(Suppl. 1); S94-98
19.. Cetani F, Frustaci G, Torregrossa L, A nonfunctioning parathyroid carcinoma misdiagnosed as a follicular thyroid nodule.: World J Surg Oncol, 2015; 13; 270
20.. Agha RA, Franchi T, Sohrabi C, The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines.: Int J Surg, 2020; 84; 226-30
21.. Freeman MN, Omar M, Shama M, Kandil E, Escaping sestamibi detection: A case report of aggressive and recurrent metastatic parathyroid carcinoma: Gland Surg, 2022; 11(6); 1111-18
22.. Asare EA, Sturgeon C, Winchester DJ, Parathyroid carcinoma: An update on treatment outcomes and prognostic factors from the National Cancer Data Base (NCDB): Ann Surg Oncol, 2015; 22(12); 3990-95
23.. Lenschow C, Schrägle S, Kircher S, Clinical presentation, treatment, and outcome of parathyroid carcinoma: Results of the NEKAR retrospective international multicenter study.: Ann Surg, 2022; 275(2); e479-e87
24.. Mohebati A, Shaha A, Shah J, Parathyroid carcinoma: Challenges in diagnosis and treatment: Hematol Oncol Clin North Am, 2012; 26(6); 1221-38
25.. McInerney NJ, Moran T, O’Duffy F, Parathyroid carcinoma: Current management and outcomes – a systematic review: Am J Otolaryngol, 2023; 44(4); 103843
26.. Agarwal G, Dhingra S, Mishra SK, Krishnani N, Implantation of parathyroid carcinoma along fine needle aspiration track: Langenbecks Arch Surg, 2006; 391(6); 623-26
27.. Spinelli C, Bonadio AG, Berti P, Cutaneous spreading of parathyroid carcinoma after fine needle aspiration cytology: J Endocrinol Invest, 2000; 23(4); 255-57
28.. Norman J, Politz D, Browarsky I, Diagnostic aspiration of parathyroid adenomas causes severe fibrosis complicating surgery and final histologic diagnosis: Thyroid, 2007; 17(12); 1251-55
29.. Paker I, Yilmazer D, Yandakci K, Intrathyroidal oncocytic parathyroid adenoma: A diagnostic pitfall on fine-needle aspiration: Diagn Cytopathol, 2010; 38(11); 833-36
30.. Sriphrapradang C, Sornmayura P, Chanplakorn N, Fine-needle aspiration cytology of parathyroid carcinoma mimic hürthle cell thyroid neoplasm: Case Rep Endocrinol, 2014; 2014; 680876
31.. Lee KM, Kim EJ, Choi WS, Intrathyroidal parathyroid carcinoma mimicking a thyroid nodule in a MEN type 1 patient: J Clin Ultrasound, 2014; 42(4); 212-14
32.. Hunter GJ, Schellingerhout D, Vu TH, Accuracy of four-dimensional CT for the localization of abnormal parathyroid glands in patients with primary hyperparathyroidism: Radiology, 2012; 264(3); 789-95
33.. Zelano L, Locantore P, Rota CA, Parathyroid carcinoma all-in-one, a rare life-threatening case with multiple systemic manifestations: Case report and review of the literature: Front Endocrinol (Lausanne), 2022; 13; 881225
34.. Hara H, Igarashi A, Yano Y, Ultrasonographic features of parathyroid carcinoma: Endocr J, 2001; 48(2); 213-17
35.. Christakis I, Vu T, Chuang HH, The diagnostic accuracy of neck ultrasound, 4D-Computed tomography and sestamibi imaging in parathyroid carcinoma: Eur J Radiol, 2017; 95; 82-88
36.. Cheon M, Choi JY, Chung JH, Differential findings of tc-99m sestamibi dual-phase parathyroid scintigraphy between benign and malignant parathyroid lesions in patients with primary hyperparathyroidism: Nucl Med Mol Imaging, 2011; 45(4); 276-84
37.. Harari A, Waring A, Fernandez-Ranvier G, Parathyroid carcinoma: A 43-year outcome and survival analysis: J Clin Endocrinol Metab, 2011; 96(12); 3679-86
38.. Merrill AL, Sims SS, Dedhia PH, Near-infrared autofluorescence features of parathyroid carcinoma.: J Endocr Soc., 2022; 6(8) bvac090
39.. Ferraro V, Sgaramella LI, Di Meo G, Current concepts in parathyroid carcinoma: A single Centre experience: BMC Endocr Disord, 2019; 19(Suppl. 1); 46
40.. Wang Q, Wang J, Xin Y, Hsa_circ_0005729 enhances accuracy in diagnosing parathyroid carcinoma.: Endocr Connect, 2022; 11(2); e210605
41.. Bae JH, Choi HJ, Lee Y, Preoperative predictive factors for parathyroid carcinoma in patients with primary hyperparathyroidism: J Korean Med Sci, 2012; 27(8); 890-95
42.. Ohkuwa K, Sugino K, Katoh R, Preoperative inflammatory markers for predicting parathyroid carcinoma: Endocr Connect, 2022; 11(7); e220062
43.. Amin MB, Greene FL, Edge SB, The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging: Cancer J Clin, 2017; 67(2); 93-99
44.. Sun XM, Pang F, Zhuang SM, Tumor size rather than the thyroid invasion affects the prognosis of parathyroid carcinoma without lymph node or distant metastasis: Eur Arch Otorhinolaryngol, 2022; 279(9); 4587-94
45.. Storvall S, Ryhänen E, Heiskanen I, MGMT promoter methylation and parathyroid carcinoma: J Endocr Soc, 2019; 3(11); 2114-22
46.. Harari A, Waring A, Fernandez-Ranvier G, Parathyroid carcinoma: A 43-year outcome and survival analysis: J Clin Endocrinol Metab, 2011; 96(12); 3679-86
47.. Chahinian AP, Holland JF, Nieburgs HE, Metastatic nonfunctioning parathyroid carcinoma: Ultrastructural evidence of secretory granules and response to chemotherapy: Am J Med Sci, 1981; 282(2); 80-84
48.. Limberg J, Stefanova D, Ullmann TM, The use and benefit of adjuvant radiotherapy in parathyroid carcinoma: A National Cancer Database Analysis: Ann Surg Oncol, 2021; 28(1); 502-11
49.. Eledreesi M, Alsubaie K, Kelany Y, Parathyroid surgery outcome at King Salman Armed Forces Hospital: Surgical Science, 2022; 13; 91-97
50.. Hussein WI, El-Maghraby TA, Al-Sanea O, Hyperfunctioning intrathyroidal parathyroid carcinoma: Saudi Med J, 2006; 27(8); 1226-29
51.. Al-Sobhi S, Ashari LH, Ingemansson S, Detection of metastatic parathyroid carcinoma with Tc-99m sestamibi imaging: Clin Nucl Med, 1999; 24(1); 21-23
52.. Al-Sobhi S, Shira H, Al-Dayel F, Parathyroid carcinoma: A report of two cases: Ann Saudi Med, 1999; 19(5); 431-33
53.. Alharbi N, Asa SL, Szybowska M, Intrathyroidal parathyroid carcinoma: An atypical thyroid lesion: Front Endocrinol (Lausanne), 2018; 9; 641
54.. Al-Maghrabi JA, Asa SL, Expression of nm23 antimetastatic gene product in parathyroid hyperplasia, adenoma and carcinoma. An immunohistological assessment.: Saudi Med J, 2005; 26(5); 728-31
55.. Al-Sulami SS, Parathyroid carcinoma: Atypical presentation and coexistence with papillary thyroid cancer: Saudi J Med Med Sci, 2015; 3; 245-46
56.. Aljabri KS, Bokhari SA, Alshareef MA, Coexistence of parathyroid cancer and papillary thyroid cancer: A case report with a review of the literature: EC Endocrinology and Metabolic Research, 2017; 1(3); 98-102
Figures
In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250