16 April 2024: Articles 
Nivolumab-Induced Cytokine Release Syndrome: A Case Report and Literature Review
Unusual clinical course, Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Adverse events of drug therapy, Educational Purpose (only if useful for a systematic review or synthesis)
Francis Ntwali1ABCDEF*, Quentin Gilliaux2A, Patrick M. Honoré1ADOI: 10.12659/AJCR.941835
Am J Case Rep 2024; 25:e941835
Abstract
BACKGROUND: CRS (cytokine release syndrome) is a massive activation of the inflammatory system characterized by a supra-physiological rate of inflammatory cytokines. The interleukin 6 cytokine plays a central role in CRS. The main clinical sign of CRS is fever, but CRS can lead to multiple organ failure in severe cases. CRS is usually described in sepsis, more recently in SARS COV-2 infection, and in chimeric antigen receptor T-cell therapy. However, it can also be associated with immune checkpoint inhibitors (ICIs), which is infrequently described. ICI have growing indications and can lead to CRS by causing an uncontrolled activation of the immune system. There are currently no treatment guidelines for ICI-induced CRS.
CASE REPORT: We report a rare case of grade 3 CRS induced by nivolumab associated with 5-fluorouracil and oxaliplatin for gastric cancer. The patient was 65-year-old man with an adenocarcinoma of the cardia. CRS developed during the tenth course of treatment and was characterized by fever, hypotension requiring vasopressors, hypoxemia, acute kidney injury, and thrombopenia. The patient was transferred quickly to the Intensive Care Unit. He was treated for suspected sepsis, but it was ruled out after multiple laboratory examinations. There was rapid resolution after infusion of hydrocortisone.
CONCLUSIONS: The use of ICIs is expanding. Nivolumab-induced CRS is rarely described but can be severe and lead to multiple organ dysfunction; therefore, intensive care practitioners should be informed about this adverse effect. More studies are needed to better understand this condition and establish treatment guidelines.
Keywords: cytokine release syndrome, Immune Checkpoint Inhibitors, Nivolumab, Receptors, Chimeric Antigen, Sepsis
Introduction
Cytokine release syndrome (CRS), or cytokine storm, can be described as a major inflammatory syndrome resulting from a supra-physiological rate of inflammatory cytokines. There is no largely accepted definition, because the line between a physiological and pathological inflammatory response is not well defined [1]. Making this differentiation is all the more difficult as some cytokines are useful to fight the invasion of pathogens and can also be harmful for the host. These inflammatory mediators are interdependent and interact in a complex manner, resulting in a massive activation of the inflammatory system [1,2].
Immune checkpoint inhibitors (ICIs) are key tools in cancer therapy, and their use is expanding. ICIs inhibit negative regulators of the immune system. By doing so, they enhance immune activity against cancer cells. Their action can lead to overstimulation of the immune system, causing immune-related adverse events and CRS. Among ICIs, programmed cell death 1 (PD-1) inhibitors and, in particular, nivolumab have been indicated for various cancer treatments. Food and Drug Administration (FDA)-approved conditions are mismatch repair deficient colorectal cancer, head and neck squamous cell carcinomas, hepatocellular carcinoma, melanoma, classic Hodgkin lymphoma, non-small-cell lung carcinoma, renal cell carcinoma, urothelial cancer, and small-cell lung carcinoma [3,4]. European Medicines Agency indications also include mesothelioma [4]. Moreover, other indications are still in trial [5].
Case Report
We report a case of cytokine release syndrome (CRS) following a combination treatment by nivolumab, 5-fluorouracil, and oxaliplatin for gastric cancer.
The patient was a 65-year-old man with a history of smoking. He was not on any daily medication. He was diagnosed with adenocarcinoma of the cardia. Extension workup showed locoregional adenopathy and liver metastases. The cancer was staged T3N3M1. The baseline C-reactive protein (CRP) level was 90 mg/L.
The patient was started on a combination therapy of nivolumab, 5-fluorouracil, and oxaliplatin. He went through the first 8 cycles of treatment with good tolerance. A few hours after the treatment cycle 9, the patient presented with fever and shivering. The next day, he was admitted in the Emergency Department with fever, shivering, tachycardia, with a heart rate of 113 beats per min, hypoxemia, with 92% SpO2, CRP level of 126 mg/dL, white blood cell count (WBC) of 4380/μL, platelet count of 117 000/μL, and creatinine level of 1.56 mg/dL. A chest radiograph showed diffuse nonspecific pulmonary infiltrates. He was hospitalized with a diagnosis of pulmonary sepsis and progressed well with antibiotic treatment by piperacilin-tazobactam. Three weeks later, in a follow-up visit, a computed tomography scan showed persistence of the pulmonary infiltrates. A diagnosis of possible diffuse interstitial lung disease was made.
During treatment cycle 10, he presented with fever, nausea, rash of the face and trunk, and hypotension. He was hospitalized in the Oncology Department and received 40 mg of methylprednisolone and 5 mg of polaramine.
The next day, he was admitted to the Intensive Care Unit (ICU) for hypotension, with an arterial pressure of 59/28 mm Hg (mean of 36 mm Hg), not responding to 2 L of normal saline infusion, tachycardia with a heart rate of 133 beats per min, and hypoxemia requiring 2 L of nasal oxygen per min. He also had a fever of 39.8°C and diarrhea. Laboratory test results showed a CRP level of 233 mg/dL, white blood cell count of 32 000/μL, platelet count of 87 000/μL, and acute kidney injury KDIGO 3, with creatinine level of 2.79 mg/dL (Figure 1).
Initial ICU treatment consisted of hemodynamic stabilization with fluid therapy and norepinephrine up to 0.14 μg/kg/min. Septic shock was suspected, and the patient was treated with broad-spectrum antibiotics (ceftazidime (2 g/24 h adapted to renal function) and vancomycin (charge dose of 25 mg/kg, followed by a continuous infusion for a plasmatic dosage of 25 to 30 mg/L) after a complete bacteriological sampling, which came back negative. The patient had an implanted port, which was removed, and bacteriological analysis was negative. Chest radio-graph showed an increase in the pulmonary infiltrate previously described, and abdominal imagery showed no abnormality. On day 4 after ICU admission, the patient was still on norepinephrine, fever was persistent, CRP level was 126 mg/dL, and platelet count continued decreasing, to 35 000/μL. Antibiotics were discontinued on day 5. On day 6, the patient received hydrocortisone of 100 mg per 24 h after a bolus dose of 100 mg. The fever ceased the same day. Norepinephrine was discontinued at day 7. On day 8, the patient was discharged from the ICU with 1 L of nasal oxygen, CRP level of 47 mg/dL, platelet count of 143 000/μL, and normalized creatinine level of 0.83 mg/dL. A chest radiograph showed a decrease of the pulmonary infiltrates. On day 15, the patient was discharged from the hospital. During a follow-up visit, the patient underwent a sensitivity test with oxaliplatine, which was negative.
Discussion
Causes of CRS
FIRST CLASSICAL CAUSE: SEPSIS:
CRS can be triggered by various conditions and therapies, including sepsis.
This dysregulation of the immune system is widely described in sepsis. The Third International Consensus Definitions Task Force for Sepsis defined sepsis as a “life-threatening organ dys-function caused by a dysregulated host response to infection” [6]. The treatment strategy for sepsis consists of organ support and pathogen elimination via administration of antibiotics.
Some studies focused on controlling the immune dysregulation in sepsis via extracorporeal hemoadsorbent devices.
A randomized controlled pilot study by Hawchar et al studied Cytosorb as a standalone treatment in septic shock. The primary endpoint was the clinical effect of the treatment. The results showed reduction in norepinephrine requirements and procalcitonin and Big-endothelin-1 concentrations in the Cytosorb group. Also, there were no related adverse events [7]. However, a randomized controlled trial in 2017 by Schädler et al studied Cytosorb in septic shock. The primary outcome was change in interleukin (IL)-6 concentration. Mortality was a secondary outcome. The results showed no reduction of serum IL-6 levels, despite up to 18% elimination by the adsorption filter [8]. This could mean that simply removing the cytokines from the serum does not treat the dysregulation, and the body reacts by making more cytokines. The results also showed no impact on mortality, organ dysfunction, and days on mechanical ventilation. The benefit of treatments targeting cytokines directly via extracorporeal hemoadsorbent devices is still unclear.
SECOND IMPORTANT CAUSE: COVID-19: COVID-19 is caused by SARS-coV-2 infection and can lead to sepsis and CRS. Nevertheless, treatment strategies are different from those in sepsis [9]. High levels of IL-6 in COVID-19 are correlated with disease severity and high mortality [10]. The RECOVERY study showed that dexamethasone reduces mortality in severe COVID-19 cases and is harmful in mild cases [11]. These results suggest that bad outcomes in severe cases of COVID-19 are due to a dysregulated immune response. This is not the case in mild cases, hence the gain from corticoids in severe cases.
LESS FREQUENT CAUSES: IMMUNOTHERAPIES, INCLUDING CHIMERIC ANTIGEN RECEPTOR T-CELL THERAPIES: There are 2 types of toxicities caused by immunotherapies: autoimmune toxicity and cytokine-associated toxicity [2]. Autoimmune toxicity relates to “on target off-tumor toxicity” and occurs when the immune system targets a specific antigen that is also expressed on healthy tissues [12]. These adverse effects are well described and there are specific management recommendations according to the organ or system affected [13]. Cytokin-associated toxicity relates to CRS and is an uncontrolled activation of the immune system unrelated to a specific antigen.
Immune effector cell-associated neurotoxicity, also known as chimeric antigen receptor (CAR)-T-cell-related encephalopathy syndrome, is a neurotoxicity associated with immunotherapy that can be associated with CRS [14]. CRS is mostly reported following CAR T-cell therapy. The incidence ranges from 57% to 93% [15]. CRS has also been reported following bispecific antibody therapy. CRS following ICI therapy has been rarely described, and factors leading to high-grade CRS are not well understood.
A 2020 report from the WHO global pharmacovigilance database Vigibase addressed 80 700 ICI-related adverse events, among which were 58 cases of CRS. The report showed that 59% of the CRS cases happened following anti-PD-1 therapies and 36% followed treatment with nivolumab [16]. In this series, CRS developed in a median of 4 weeks after ICI initiation. Severity of the cases was not graded, but 17% of the CRS cases were considered life-threatening. Positive outcome after CRS was associated with younger age (<65 years), mono-therapy, and prolonged treatments [14].
Tay et al published a case series of 25 patients who developed CRS after ICI therapy. Among the 25 cases, 68% involved anti-PD-1 therapies. High-grade CRS (grade 3 and above) occurred in 32% of patients. In this series, CRS developed in a median of 11 days after ICI initiation [17]. No association was made between patient characteristics and outcome.
As mentioned above, indications for ICI are expanding. Moreover, new molecules are being studied [18]. These treatments are often used in combination with other cancer therapies to improve efficacy. However, the combination can increase the risk of immune-related adverse events [19]. And as shown by Ceschi et al, monotherapy is a good prognostic factor in ICI-induced CRS [16]. Since more patients are being treated with immunotherapies and ICI, intensive care practitioners should be informed about their adverse effects, especially
CRS, given that patients presenting with a CRS grade above 3 need to be treated in the ICU (Table 1).
SIGNS AND SYMPTOMS OF CRS:
A wide range of signs and symptoms can be observed in CRS. Fever (core temperature above 38°C) is a key clinical sign and is present in the vast majority of CRS cases [1,2]. Fever is present in the beginning of CRS and can be high, up to 40.5°C. Mild forms of CRS can be accompanied by fatigue, headaches, rash, diarrhea, and arthralgia. Severe forms can be accompanied by any organ dysfunction: hypotension, acute renal failure, acute respiratory distress syndrome, cardiac dysfunction, and shock, requiring quick and appropriate ICU treatment.
As discussed above, severe CRS typically presents with fever and organ dysfunction. The first diagnosis to rule out is septic shock, since in this case, antibiotics should be started immediately [20]. CRS diagnosis is mainly based on signs and symptoms, so it should be evoked as soon as other obvious diagnostic hypothesis are ruled out according to the clinical context. In the context of cancer therapy, CRS diagnosis should be evoked in cases of fever occurring after treatment with CAR-T cells, but also with ICIs, particularly nivolumab. Time to onset is variable. In our case, fever occurred 140 days after nivolumab initiation and minutes after the last dose. In the series published by Tay et al, CRS developed in a median of 11 days after ICI initiation [17]. In the series published by Ceschi et al using the WHO global pharmacovigilance database, the authors found a median of 4 weeks [16]. We retrieved 12 cases of nivolumab-induced CRS and created our own case series, in which the median was 8 days (Table 2).
LABORATORY FINDINGS:
Routine laboratory findings are unspecific, are the markers of a systemic inflammatory response syndrome [2], and include high CRP levels, leukopenia/leucocytosis, thrombopenia, altered liver and renal test results, and coagulopathy. Chemotherapy or the underlying cancer can induce leukopenia, neutropenia, and thrombopenia. CRS can also overlap with a tumor lysis syndrome (laboratory findings include hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia) and with macrophage activation syndrome (laboratory findings include hyperferritinemia and hypertriglyceridemia) [2,33].
Cytokines are helpful diagnostic and severity markers, but their testing is unavailable in most hospitals. However, serum reveals a high level of circulating cytokines, including IL-6, IL-10, IFNα, and IL1β. IL-6 is a cytokine with pleiotropic inflammatory and anti-inflammatory effects and has a central role in CRS [2]. Baseline cytokine values can be elevated in several disorders and cancer. Therefore, the increase in cytokine levels could be a better marker than the absolute value [1,2]. CRP, which is produced by the liver following the rise of IL-6 levels, could be a good surrogate to IL-6 testing [34].
GRADING CRS:
The American Society for Transplantation and Cellular Therapy (ASTCT) published guidelines on grading CRS caused by CAR T-cells therapy [35], including 4 grades. Fever is a mandatory parameter in all grades. Patients presenting with grade 3 CRS or above will generally be taken care of in an ICU because they will have hypotension requiring vasopressors or hypoxemia requiring high-flow oxygen or oxygen masks (Table 3).
CRS from other causes is graded by the Common Terminology Criteria for Adverse Events (CTCAE) [36], in which there are 5 grades (grade 5 is death). Similar to the ASTCT guidelines, patients with CTCAE grade 3 CRS or above will generally be taken care of in an ICU (Table 1).
TREATMENT OF CRS:
At this time, there are no treatment guidelines for ICI-induced CRS. In 2020, the Society for Immunotherapy of Cancer released guidelines on CAR T-cell-induced CRS [18]. The recommendations consist of tocilizumab (8 mg/kg) and methylprednisolone (2 mg/kg). Dexamethasone (0.5 mg/kg) is used in case of neurological symptoms because of its good blood-brain barrier penetration. If a patient presents with grade 2 CRS or above, the recommended treatment is 1 dose of tocilizumab. In the absence of improvement, it is recommended that the patient receive corticoid treatment and 1 supplementary dose of tocilizumab.
IL-6 is a central mediator in CRS. Therapy targeting IL-6 has proven to be effective in CAR T-cell-induced CRS [37]. Tocilizumab is a humanized monoclonal antibody that competitively inhibits binding of IL-6 to its receptor. Tocilizumab was approved by the FDA in 2017 for the treatment of CAR T-cell-induced CRS and in 2022 for the treatment of severe forms of COVID-19 [38]. Common adverse effects of tocilizumab include infections, neutropenia, hypercholesterolemia, and liver test disturbances [39].
Corticosteroids are not recommended as a first line therapy, because they have pleiotropic effects on the immune system. Corticoids have been shown to inhibit IL-1, IL-6, IL-8, tumor necrosis factor (TNF) alpha, TNF receptors 1 and 2, and nitric oxide formation [40]. There is concern that they would have negative effects on the CAR-infused T cells. Meanwhile, the evidence for these negative effects is poor, and some authors are in favor of initial bitherapy with tocilizumab and glucocorticoids in cases of life-threatening CRS induced by ICIs [2].
Common adverse effects of methylprednisolone and dexamethasone include immune suppression, decreased resistance to infection, hyperglycemia, and secondary adrenal insufficiency [41]. Tocilizumab is an expensive drug, and its availability can be limited in some settings, while corticoids are widely available and can be used rapidly in case of emergency.
They are no treatment guidelines for ICI-induced CRS. In our case, CRS was successfully treated with hydrocortisone. We used 100 mg of hydrocortisone daily after a bolus of 100 mg, to accelerate the resolution of shock, as recommended by the Surviving Sepsis Campaign guidelines for the treatment of septic shock with ongoing requirement of vasopressors [20]. At this point, CRS was not evoked, and the patient was treated as a patient with septic shock. The Surviving Sepsis Campaign guidelines recommend the dose of 200 mg of hydrocortisone daily, but the patient improved so dramatically that we continued with the lower dosage. Given the patient’s clinical improvement, tocilizumab was not needed to treat the CRS. In the series published by Tay et al, 36% of patients received methylprednisolone, 4% received dexamethasone for neurologic toxicities, and 24% received tocilizumab. Among patients who were treated with tocilizumab, 83% had severe CRS (grade above 3) [17]. In our case series, all the patients were treated with corticoids in a variety of regimens, except for 1 case in which treatment was not specified. Four patients received tocilizumab, 75% of whom had severe CRS (Table 2).
More studies are needed to establish which patients can be treated with corticoids and which patients will require tocilizumab, especially since we cannot predict which patients will develop severe CRS. Also, on admission in the ICU, our patient was treated for suspected sepsis and not for CRS. Cytokine testing was not performed, as it is not done routinely. This testing would have been useful to help with diagnosis, treatment monitoring, and better understanding of this condition.
Conclusions
The use of ICIs is expanding. Among ICIs, nivolumab has growing indications. ICI-induced CRS, particularly nivolumab-induced CRS, can be severe and lead to multiple organ dysfunction; therefore, intensive care practitioners should be informed about this adverse effect. More studies are needed to better understand this condition and to determine risk factors of severe CRS. The patient in our case was successfully treated with hydrocortisone; however, treatment guidelines are needed to improve clinical management.
Tables
Table 1.. Cytokine release syndrome grading according to the Common Terminology Criteria for Adverse Events.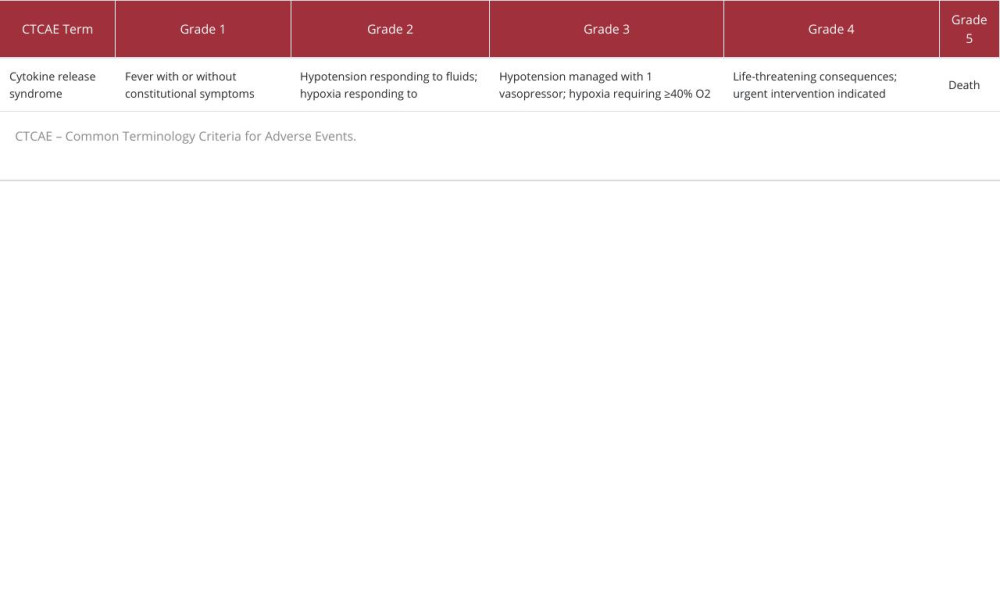 Table 2.. Case reports on nivolumab-induced cytokine release syndrome.
Table 2.. Case reports on nivolumab-induced cytokine release syndrome.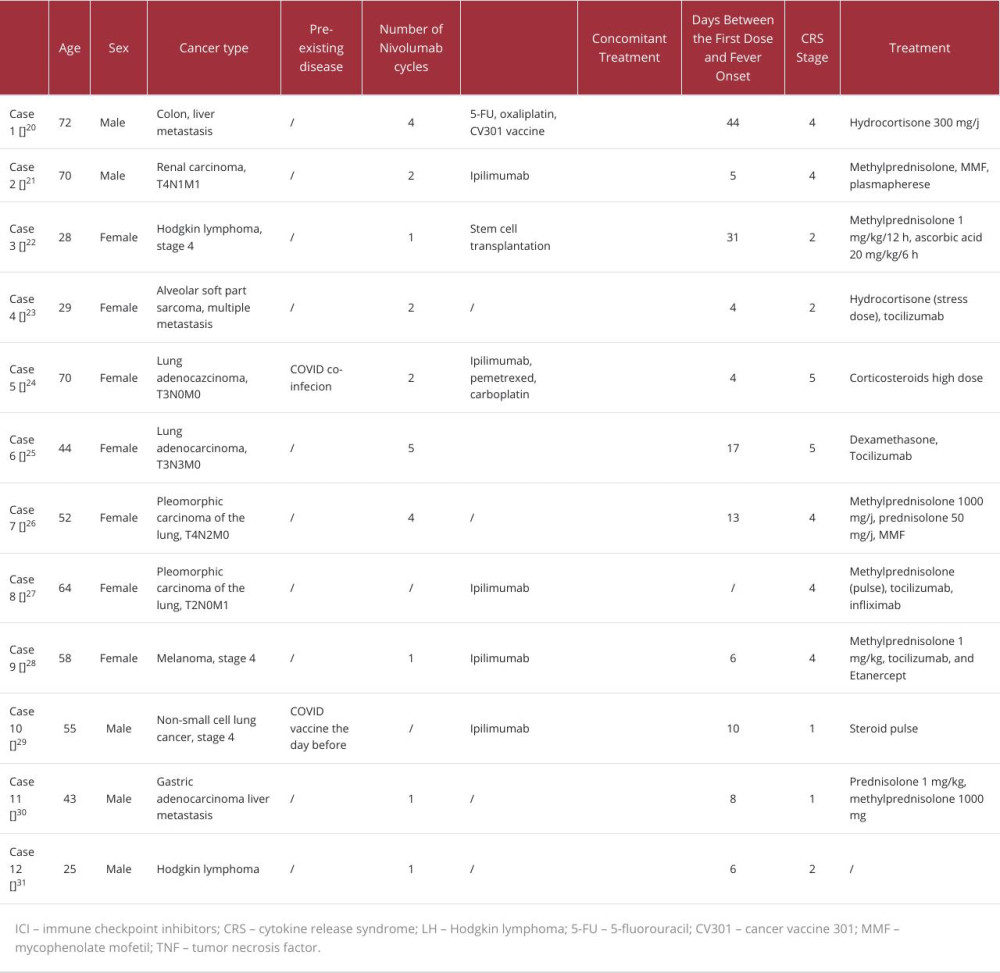 Table 3.. Cytokine release syndrome grading according to the American Society for Transplantation and Cellular Therapy.
Table 3.. Cytokine release syndrome grading according to the American Society for Transplantation and Cellular Therapy.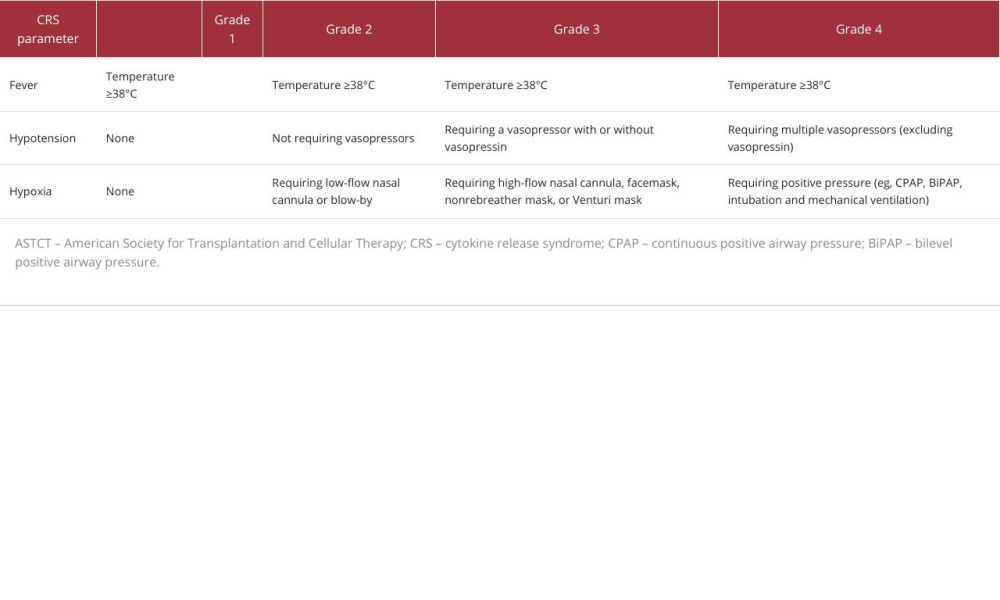
References:
1.. Fajgenbaum DC, June CH, Cytokine storm: N Engl J Med, 2020; 383(23); 2255-73
2.. Lee DW, Gardner R, Porter DL, Current concepts in the diagnosis and management of cytokine release syndrome: Blood, 2014; 124(2); 188-95
3.. , Prescribing information for OPDIVO Last updated in 2021 July [cited 2023 Jun. 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125554s081lbl.pdf
4.. , Opdivo Last updated in 2023 March [cited 2023 Jun. 1]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo#assessment-history-section
5.. Naimi A, Mohammed RN, Raji A, Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons: Cell Commun Signal, 2022; 20(1); 44
6.. Singer M, Deutschman CS, Seymour CW, The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3): JAMA, 2016; 315(8); 801-10
7.. Hawchar F, László I, Öveges N, Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study: J Crit Care, 2019; 49; 172-78
8.. Schädler D, Pausch C, Heise D, The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial: PLoS One, 2017; 12(10); e0187015
9.. Alhazzani W, Evans L, Alshamsi F, Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: First update: Crit Care Med, 2021; 49(3); e219-e34
10.. Lucas C, Wong P, Klein J, Longitudinal analyses reveal immunological misfiring in severe COVID19: Nature, 2020; 584(7821); 463-69
11.. Horby P, Lim WS, Emberson JR, Dexamethasone in hospitalized patients with COVID-19: N Engl J Med, 2021; 384(8); 693-704
12.. Fishman JA, Hogan JI, Maus MV, Inflammatory and infectious syndromes associated with cancer immunotherapies: Clin Infect Dis, 2019; 69(6); 909-20
13.. Gutierrez C, McEvoy C, Munshi L, Critical care management of toxici-ties associated with targeted agents and immunotherapies for cancer: Crit Care Med, 2020; 48(1); 10-21
14.. Porter DL, Maloney DG, Cytokine release syndrome (CRS): UpToDate, Wolters Kluwer Accessed on 2023 Feb 19
15.. Santomasso BD, Nastoupil LJ, Adkins S, Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline: J Clin Oncol, 2021; 39(35); 3978-92 [Erratum in: J Clin Oncol. 2022;40(8):919]
16.. Ceschi A, Noseda R, Palin K, Verhamme K, Immune checkpoint inhibitor-related cytokine release syndrome: Analysis of WHO global pharmacovigilance database: Front Pharmacol, 2020; 11; 557
17.. Tay SH, Toh MMX, Thian YL, Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: A case series of 25 patients and review of the literature: Front Immunol, 2022; 13; 807050
18.. Maus MV, Alexander S, Bishop MR, Society for immunotherapy of cancer (SITC) clinical practice guideline on immune effector cell-related adverse events: J Immunother Cancer, 2020; 8(2); e001511
19.. Naimi A, Mohammed RN, Raji A, Tumor immunotherapies by immune checkpoint inhibitors (ICIs); The pros and cons: Cell Commun Signal, 2022; 20(1); 44
20.. Evans L, Rhodes A, Alhazzani W, Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021: Intensive Care Med, 2021; 47(11); 1181-47
21.. Ciner AT, Hochster HS, August DA, Delayed cytokine release syndrome after neoadjuvant nivolumab: A case report and literature review: Immunotherapy, 2021; 13(13); 1071-78
22.. Ohira J, Kawamoto M, Sugino Y, Kohara N, A case report of fulminant cytokine release syndrome complicated by dermatomyositis after the combination therapy with immune checkpoint inhibitors: Medicine (Baltimore), 2020; 99(15); e19741
23.. Sindel A, Taylor T, Chesney A, Hematopoietic stem cell mobilization following PD-1 blockade: Cytokine release syndrome after transplantation managed with ascorbic acid: Eur J Haematol, 2019; 103(2); 134-36
24.. Rotz SJ, Leino D, Szabo S, Severe cytokine release syndrome in a patient receiving PD-1-directed therapy: Pediatr Blood Cancer, 2017; 64(12); 26642
25.. Murata D, Azuma K, Tokisawa S, A case of cytokine release syndrome accompanied with COVID-19 infection during treatment with immune checkpoint inhibitors for non-small cell lung cancer: Thorac Cancer, 2022; 13(20); 2911-14
26.. Deng PB, Jiang J, Hu CP, Tumor-related cytokine release syndrome in a treatment-naïve patient with lung adenocarcinoma: A case report: World J Clin Cases, 2022; 10(5); 1580-85
27.. Honjo O, Kubo T, Sugaya F, Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: A case report: J Immunother Cancer, 2019; 7(1); 97
28.. Kunimasa K, Inoue T, Matsueda K, Cytokine release syndrome and immune-related pneumonitis associated with tumor progression in a pulmonary pleomorphic carcinoma treated with nivolumab plus ipilimumab treatment: A case report: JTO Clin Res Rep, 2021; 3(2); 100272
29.. Menakuru SR, Azeem Q, Priscu A, Stage 4 cytokine release syndrome caused by the first dose of nivolumab and ipilimumab combination therapy in a patient with metastatic melanoma successfully treated with methylprednisolone, tocilizumab, and etanercept: Case Rep Oncol, 2022; 15(2); 648-53
30.. Sumi T, Koshino Y, Michimata H, Cytokine release syndrome in a patient with non-small cell lung cancer on ipilimumab and nivolumab maintenance therapy after vaccination with the mRNA-1273 vaccine: A case report: Transl Lung Cancer Res, 2022; 11(9); 1973-76
31.. Oda H, Ishihara M, Miyahara Y, First case of cytokine release syndrome after nivolumab for gastric cancer: Case Rep Oncol, 2019; 12(1); 147-56
32.. Zhao L, Yang Y, Li W, Nivolumab-induced cytokine-release syndrome in relapsed/refractory Hodgkin’s lymphoma: A case report and literature review: Immunotherapy, 2018; 10(11); 913-17
33.. Michot JM, Lazarovici J, Tieu A, Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage?: Eur J Cancer, 2019; 122; 72-90
34.. Hidayat F, Labeda I, Sampetoding S, Correlation of interleukin-6 and C-reactive protein levels in plasma with the stage and differentiation of colorectal cancer: A cross-sectional study in East Indonesia: Ann Med Surg (Lond), 2021; 62; 334-40
35.. Lee DW, Santomasso BD, Locke FL, ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells: Biol Blood Marrow Transplant, 2019; 25(4); 625-38
36.. , Common Terminology Criteria for Adverse Events (CTCAE) Last updated 2021 Apr 19 [cited 2023 Jun. 1]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50
37.. Kotch C, Barrett D, Teachey DT, Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome: Expert Rev Clin Immunol, 2019; 15(8); 813-22
38.. , Frequently asked question on the emergency use authorisation of Actemra (Tocilizumab) for treatment of COVID-19 Dec 21, 2002 [cited 2023 Jun. 1]. Available from: https://www.fda.gov/media/150345/download
39.. Sheppard M, Laskou F, Stapleton PP, Tocilizumab (Actemra): Hum Vaccin Immunother, 2017; 13(9); 1972-88
40.. Keh D, Boehnke T, Weber-Cartens S, Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: A double-blind, randomized, placebo-controlled, crossover study: Am J Respir Crit Care Med, 2003; 167(4); 512-20
41.. , PubChem Compound Summary for CID 6741, Methylprednisolone [cited 2023 Aug. 30]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Methylprednisolone
Tables
 Table 1.. Cytokine release syndrome grading according to the Common Terminology Criteria for Adverse Events.
Table 1.. Cytokine release syndrome grading according to the Common Terminology Criteria for Adverse Events. Table 2.. Case reports on nivolumab-induced cytokine release syndrome.
Table 2.. Case reports on nivolumab-induced cytokine release syndrome. Table 3.. Cytokine release syndrome grading according to the American Society for Transplantation and Cellular Therapy.
Table 3.. Cytokine release syndrome grading according to the American Society for Transplantation and Cellular Therapy. Table 1.. Cytokine release syndrome grading according to the Common Terminology Criteria for Adverse Events.
Table 1.. Cytokine release syndrome grading according to the Common Terminology Criteria for Adverse Events. Table 2.. Case reports on nivolumab-induced cytokine release syndrome.
Table 2.. Case reports on nivolumab-induced cytokine release syndrome. Table 3.. Cytokine release syndrome grading according to the American Society for Transplantation and Cellular Therapy.
Table 3.. Cytokine release syndrome grading according to the American Society for Transplantation and Cellular Therapy. In Press
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








