12 July 2020: Articles 
Pneumatosis Intestinalis and Hepatic Portal Venous Gas: Watch and Wait or Emergency Surgery? A Case Report and Literature Review
Challenging differential diagnosis, Management of emergency care, Rare coexistence of disease or pathology
Rigers Dibra1ABEF, Arcangelo Picciariello1ABEF*, Giuseppe Trigiante1AF, Grazia Labellarte1AF, Giovanni Tota1AF, Vincenzo Papagni1ABEF, Gennaro Martines1AF, Donato F. Altomare1AEFDOI: 10.12659/AJCR.923831
Am J Case Rep 2020; 21:e923831
Abstract
BACKGROUND: Hepatic portal venous gas (HPVG) associated with pneumatosis intestinalis (PI) can be indicative of several diseases, including inflammatory bowel disease (IBD), infective and obstructive gastrointestinal conditions, and also potentially life-threatening situations such as mesenteric ischemia.
CASE REPORT: A 60-year-old female patient came to our attention with evidence at computed tomography (CT) scan of gas in the portal vein and bowel walls with no sign of ischemia. General tenderness of the abdomen with absence of bowel sounds was detected at the physical examination. An exploratory laparotomy was performed with evidence of mesenteric ischemia.
CONCLUSIONS: Emergency surgery should be indicated when CT signs of PI and HPVG occur along with a clinical situation strongly suggestive of bowel ischemia, even with no radiological sign of this critical condition.
Keywords: Emergency Treatment, Laparotomy, Pneumatosis cystoides intestinalis, Portal Vein, Embolism, Air, Emergencies, mesenteric ischemia, Tomography, X-Ray Computed
Background
Pneumatosis intestinalis (PI) described by Du Vernoy in 1730 is a rare condition that refers to the presence of multiple gaseous cysts in the intestinal submucosa and subserosa with a reported incidence of 0.03%. It can be divided in a benign and in a life-threatening condition requiring surgery [1].
The radiographic incidence of PI has been reported to be up to 0.37% of patients who have abdominal computed tomography (CT) scans [2]. PI can be divided into primary/idiopathic type (15%), which refers to air pockets that imply to a chronic and benign idiopathic etiology, and secondary type (85%), which refers to radiological findings of linear, microvesicular, or more circumferential appearing intramural gas caused by several predisposing factors [3]. The peak age at onset is 45.3±15.6 years with a male to female ratio of 2.4: 1 [4].
Hepatic portal venous gas (HPVG) described on neonates by Wolfe is a radiological sign defined as tubular areas of decreased attenuation in the liver periphery [5,6]. Its incidence is still unclear, and it may occur when intraluminal gas from the intestine or gas produced by certain bacteria migrates into the porto-mesentric venous circulation [7].
Several diseases can underlie PI and HPVG such as Crohn disease, ulcerative colitis, graft versus host disease, bowel obstruction, pseudo-obstruction, bacterial abscesses, diverticulitis, paralytic ileus, suppurative cholangitis, and colo-venous fistulae [8]. Pneumatosis intestinalis and HPVG can be associated in 60% to 80% of cases and the best strategy for the treatment of this condition is still debated [9]. In fact, several conservative treatments have been proposed and surgery should be considered when radiological and/or clinical findings demonstrated an emergency scenario such as mesenteric ischemia and bowel obstruction. The etiology of these conditions can be multifactorial and should be investigated in order to carry out the best treatment for the patient. We aim to report our experience in the management of a patient with PI and HPVG who underwent exploratory laparotomy with the evidence of a mesenteric ischemia that was not detected at the CT scan.
Case Report
A 60-year-old female was admitted to our Unit showing tenderness of the abdomen and epigastric pain and Glasgow coma scale (GCS) score of 15. Patient referred fever, weight loss, diarrhea (5–6 discharges/day) and lack of appetite in the last week. No other comorbidities or previous surgeries were reported. At physical examination (weight 35 kg, height 150 cm, body mass index 15.5 kg/m2) Blumberg sign was negative but a general tenderness with absence of bowel sounds was detected.
On presentation, the patient was hemodynamically stable and she had a temperature of 39°C. Her vital signs were blood pressure (BP) 140/85 mmHg, heart rate (HR) 99 beats per minute (bpm), respiratory rate (RR) 22 breaths per minutes. The hemogas analysis showed metabolic acidosis (pH 7.14, HCO3: 16 mEq/L). Blood tests showed neutrophilic leukocytosis (white blood cell [WBC] 14.51 x 103/uL, neutrophil granulocytes 86.4%), microcytic anemia (hemoglobin 8.2 g/dL, hematocrit 25%, mean corpuscular volume [MCV] 72 fL) with a slight hypercalcemia (1.29 mmol/L), hyperchloremia (118 mmol/L) and an increase of C-reactive protein (182 mg/L); lactic acid was 1.3 mmol/L.
At First Aid assessment an electrocardiogram (ECG) was performed (sinus rhythm, 87 bpm) and the patient underwent chest and abdomen x-ray showing multiple and diffuse air-fluid levels with overdistention of intestinal loops, suggestive for mechanical ileum. A CT scan with contrast medium was performed, demonstrating distension of the stomach and of all loops of the small intestine that appeared vital but over-stretched due to the presence of fluid material. Bowel walls had signs of slight parietal pneumatosis (Figure 1). Portal venous gas was detected (Figure 2).
Considering the clinical condition (tenderness of the abdomen, paralytic ileus and increased level of lactate: 3.2 mmol/L at 6 hours after the first measurement) was suggestive of an intestinal ischemia, despite the absence of signs of ischemia on CT scan, the patient underwent emergency surgery (ASA III E). During the operation, the abdomen was explored with the evidence of widespread relaxation of the loops of the small intestine that appeared necrotic at the pelvic level (Figure 3). The approach by laparotomy was preferred because of the high distension of small intestine loops demonstrated on the CT-scan. No free liquid and air were identified and the small bowel in the pelvis had clear signs of venous congestion, edema, and ischemia but despite CT images, no signs of macroscopically evident intestinal pneumatosis were visible. The resection of 1.5 meters of small bowel was performed with direct side to side anastomosis between jejunum and distal ileum. One drain was placed in the pelvis. The patient underwent antibiotic and fluid replacement therapy and she was discharged on postoperative day 8 with no postoperative or wound complication and with nutritional support to prevent a malabsorption disorder due to the short bowel syndrome. A blood test to check electrolytes was suggested 1 week after the patient’s discharge. She referred diarrhea for 2 weeks and she was followed-up by a gastroenterologist.
Macroscopic pathological finding revealed a small intestine segment of about 150 cm of uniformly brownish complexion, edematous wall with necrotic-inflammatory and hemorrhagic phenomena characterized by submucosal gas cysts. The histo-pathology the specimen was characterized by serous fluid with inflammatory cell infiltrate with no evidence of thrombosis.
Discussion
Hepatic portal venous gas and pneumatosis intestinalis are rare conditions and the management of their association is rarely discussed in the literature. Most patients affected by PI are aged between 50 and 80 years old and are asymptomatic, with a reported incidence of 0.03% [3,10].
PI usually occurs with non-specific symptoms such as abdominal pain, constipation, diarrhea, weight loss, hematochezia, and tenesmus. Some of the potential underlying conditions include vascular pathologies such as intestinal infarction and mesenteric ischemia or chronic conditions such as chronic obstructive pulmonary disease and chronic immunosuppression but also other causes such as intestinal obstruction and toxic megacolon [11].
The localization of PI can be in different parts of the gastrointestinal tract: colon (46%) small intestine (27%), both large and small intestine (7%), and stomach (5%). Usually, the prognosis of PI is good, but it is mandatory to exclude negative predictive factors such as associated HPVG, altered values on blood tests, pH value <7.3, bicarbonate level <20 ml/L, amylase level >200 U/L, and a lactate >2 mmol/L [12].
Several pathogenetic hypothesis have been elaborated to explain PI including mechanical, bacterial, biochemical, and pulmonary theories. The mechanical theory postulates a dysfunction of the intestinal mucosa through which the gas diffuses into the wall and along the blood vessels. The bacterial theory is explained by a diffusion of gas produced by bacteria into the submucosa through lesions of the luminal mucosa. Furthermore, the high production of hydrogen by some bacteria present in the intestine led to an increased pressure that allows a spread in the submucosal space, where they gather to form bubbles. Finally, pulmonary theory traces the origin of PI to the alveolar rupture and diffusion of gas along the bronchopulmonary bundles and subsequently to the mediastinum and retroperitoneum up to the mesenteric vessels [13]. In the literature there are also described cases in which PI is caused by intake of some drugs including alpha-glucosidase inhibitors (α-GI) that may cause a fermentation of carbohydrates, an increased intraluminal pressure with gases which break through the integrity of the mucosa [12,14]. Another rare cause of PI may be the presence of misunderstood malignant neoplasms, with a not clarified pathogenetic mechanism [15].
HPVG can be caused by a migration of gas from the bowel lumen, in case of abscess or the presence of gas-forming organisms in the portal venous system. Sometimes, when associated with
PI, HPVG can be iatrogenic especially in conditions of malnutrition [16]. This condition is associated with a high mortality rate (56–90%) and may require an urgent operative intervention [17].
The most accurate examination for the diagnosis of PI remains the abdomen CT scan, even if other imaging techniques such as x-ray of the abdomen, barium enema studies, ultrasound (US) and magnetic resonance imaging (MRI) may result useful for a differential diagnosis [18]. The usual aspect of PI at CT scan is the presence of bubbles or cysts within the bowel wall.
The CT scan evidence of colic pneumatosis is not always suggestive of mesenteric ischemia [2]. However, the association between PI and porto-mesenteric gas can be related to a higher incidence of intestinal infarction [19].
Wayne et al. tried to develop an algorithm for the management of PI and HPVG. They found that patients with CT scan evidence of pneumatosis intestinalis, and portal venous gas should undergo surgery when a “mechanical” cause (previous surgery, abdominal hernia, volvulus) or mesenteric ischemia (in patients with coronary artery disease and other cardiovascular diseases) were identified [20].
The choice of treatment depends on patient’s clinical presentation, on the imaging, and on laboratory evidences. Usually, several conservative treatments such as the administration of antibiotics and sclerotherapy or hyperbaric oxygen can be proposed as alternative or before surgery [21].
However, pneumatosis and porto-mesenteric venous gas may not be burdened by an unfavorable outcome when associated with several non-ischemic conditions, such as inflammatory, iatrogenic, traumatic, infectious, neoplastic, obstructive, and idiopathic causes [22]. Some reports have suggested that even in cases of pneumoperitoneum with PI at CT scan, an emergency operation is not indicated if the patient is clinically stable [23–25].
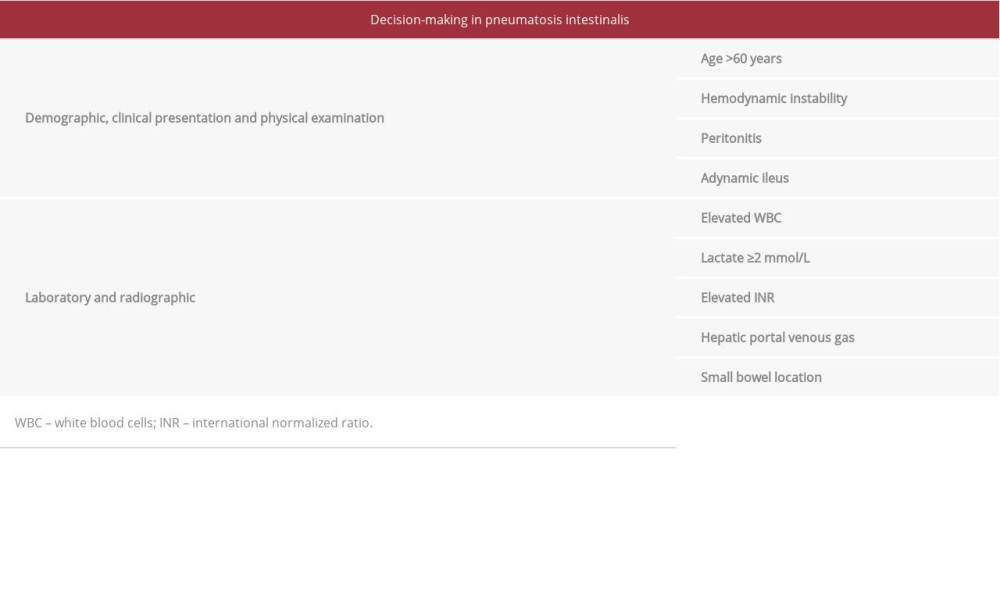
A comprehensive review by Torres et al. found that age over 60, abdominal stiffness, altered lactate levels, and localization at the small bowel were potential conditions of life-threatening scenarios in patients with PI [26].
In our case, there was no evidence of mesenteric ischemia but the association of PI and HPVG, the clinical signs of the patient with paralytic ileus, and the increased level of lactate, influenced our decision-making process and we performed an emergency laparotomy. In fact, according to the literature, primary operative indications should be considered when there are obstructive symptoms, high WBC, CT findings of HPVG, and patients older than 60 years old; while secondary indications include sepsis and/or acidosis with lactate >2 mmol/L demonstrated [12,27].
In a recent Predictive Evaluation Study from the American Association for the Surgery of Trauma, the adynamic ileus, when suspecting ischemia, is an ominous sign indicating progression to necrosis especially when associated with a high level of lactate. Conversely, radiological patterns did not appear to be as useful in distinguishing pathologic PI from benign PI in the context of other independent predictors. Furthermore, the decreased level of hemoglobin, could reflect a sloughing of the mucosa as an effect of necrosis which leads to gastrointestinal bleeding [28] (Table 1).
We speculated that the pathogenesis of this case could be explained by the mechanical theory aforementioned. Considering the absence of a clear sign of vessel obstruction at the CT-scan, the intraoperative scenario and the histopathological finding on the resected specimen we could suspected a nonocclusive mesenteric ischemia leading to damage of the small bowel wall [29,30].
Conclusions
The manifestation of PI associated with HPVG can include symptoms ranging from mild abdominal discomfort and diarrhea up to peritonitis. Surgical treatment is not generally indicated in the presence of only radiological evidence of PI and HPVG if the patient is clinically stable. Emergency surgery should be performed when CT scan demonstrate PI and HPVG with no evidence of ischemia is associated with a clinical presentation of patient strongly suggestive of bowel ischemia.
References:
1.. Di Pietropaolo M, Trinci M, Giangregorio C, Pneumatosis cystoides intestinalis: Case report and review of literature: Clin J Gastroenterol, 2020; 13(1); 31-36
2.. Morris MS, Gee AC, Cho SD, Management and outcome of pneumatosis intestinalis: Am J Surg, 2008; 195(5); 679-82 ; discussion 682–83
3.. Wang YJ, Wang YM, Zheng YM, Pneumatosis cystoides intestinalis: Six case reports and a review of the literature: BMC Gastroenterol, 2018; 18(1); 100
4.. Wu LL, Yang YS, Dou Y, Liu QS, A systematic analysis of pneumatosis cystoids intestinalis: World J Gastroenterol, 2013; 19(30); 4973-78
5.. Sebastia C, Quiroga S, Espin E, Portomesenteric vein gas: pathologic mechanisms, CT findings, and prognosis: Radiographics, 2000; 20(5); 1213-24 ; discussion 1224–26
6.. Wolfe JN, Evans WA, Gas in the portal veins of the liver in infants; A roentgenographic demonstration with postmortem anatomical correlation: Am J Roentgenol Radium Ther Nucl Med, 1955; 74(3); 486-88
7.. Hou SK, Chern CH, How CK, Hepatic portal venous gas: Clinical significance of computed tomography findings: Am J Emerg Med, 2004; 22(3); 214-18
8.. Kinoshita H, Shinozaki M, Tanimura H, Clinical features and management of hepatic portal venous gas: Four case reports and cumulative review of the literature: Arch Surg, 2001; 136(12); 1410-14
9.. Sooby P, Harshen R, Joarder R, An unusual triad of pneumatosis intestinalis, portal venous gas and pneumoperitoneum in an asymptomatic patient: J Surg Case Rep, 2015; 2015(4); rjv305
10.. Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA, Pneumatosis intestinalis: A review: Am J Gastroenterol, 1995; 90(10); 1747-58
11.. Sanford Z, Brown S, Tran MN, Updates on the utility of diagnostic laparoscopy in the management of pneumatosis intestinalis: An improvement to the current treatment algorithm: Surg Innov, 2018; 25(6); 648-50
12.. Ling F, Guo D, Zhu L, Pneumatosis cystoides intestinalis: A case report and literature review: BMC Gastroenterol, 2019; 19(1); 176
13.. Ha D, Tsai CJ: BMJ Case Rep, 2012; 2012; bcr2012006720
14.. Police A, Charre L, Volpin E, Pneumatosis cystoides intestinalis induced by the alpha-glucosidase inhibitor complicated from sigmoid volvulus in a diabetic patient: Int J Colorectal Dis, 2020; 35(5); 943-46
15.. Liu T, Zhang S, Mao H, Gastrointestinal malignant neoplasms disguised as pneumatosis cystoids intestinalis: A case report and literature review: Medicine (Baltimore), 2017; 96(51); e9410
16.. Perez Rivera CJ, Ramirez NA, Gonzalez-Orozco A, Pneumoperitoneum, pneumatosis intestinalis and portal venous gas: Rare gastrostomy complications case report: Int J Surg Case Rep, 2019; 58; 174-77
17.. Abboud B, El Hachem J, Yazbeck T, Doumit C, Hepatic portal venous gas: Physiopathology, etiology, prognosis and treatment: World J Gastroenterol, 2009; 15(29); 3585-90
18.. Pear BL, Pneumatosis intestinalis: A review: Radiology, 1998; 207(1); 13-19
19.. Wiesner W, Mortele KJ, Glickman JN, Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: Correlation of CT findings with severity of ischemia and clinical outcome: Am J Roentgenol, 2001; 177(6); 1319-23
20.. Wayne E, Ough M, Wu A, Management algorithm for pneumatosis intestinalis and portal venous gas: Treatment and outcome of 88 consecutive cases: J Gastrointest Surg, 2010; 14(3); 437-48
21.. Dhadlie S, Mehanna D, McCourtney J, Pneumatosis intestinalis a trap for the unwary: Case series and literature review: Int J Surg Case Rep, 2018; 53; 214-17
22.. Zhang D, Weltman D, Baykal A, Portal vein gas and colonic pneumatosis after enema, with spontaneous resolution: Am J Roentgenol, 1999; 173(4); 1140-41
23.. Zhang H, Jun SL, Brennan TV, Pneumatosis intestinalis: Not always a surgical indication: Case Rep Surg, 2012; 2012; 719713
24.. St Peter SD, Abbas MA, Kelly KA, The spectrum of pneumatosis intestinalis: Arch Surg, 2003; 138(1); 68-75
25.. Knechtle SJ, Davidoff AM, Rice RP, Pneumatosis intestinalis. Surgical management and clinical outcome: Ann Surg, 1990; 212(2); 160-65
26.. Torres US, Fortes C, Salvadori PS, Pneumatosis from esophagus to rectum: A comprehensive review focusing on clinico-radiological differentiation between benign and life-threatening causes: Semin Ultrasound CT MR, 2018; 39(2); 167-82
27.. Greenstein AJ, Nguyen SQ, Berlin A, Pneumatosis intestinalis in adults: Management, surgical indications, and risk factors for mortality: J Gastrointest Surg, 2007; 11(10); 1268-74
28.. Ferrada P, Callcut R, Bauza G, Pneumatosis intestinalis predictive evaluation study: A multicenter epidemiologic study of the American Association for the Surgery of Trauma: J Trauma Acute Care Surg, 2017; 82(3); 451-60
29.. Dhoble A, Patel K, Khasnis A, Non-occlusive mesenteric ischemia leading to ‘pneumatosis intestinalis’: A series of unfortunate hemodynamic events: Cases J, 2008; 1(1); 60
30.. Hohmann C, Teuteberg S, Aschenbrenner I, [Non-occlusive mesenteric ischemia caused by diabetic ketoacidosis – pneumatosis intestinalis and portal venous gas as an indication of mesenteric ischemia]: Dtsch Med Wochenschr, 2019; 144(23); 1638-41 [in German]
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250