17 July 2020: Articles 
Infected Hemodialysis Arteriovenous Fistula with Distant Explosive Pleuritis: A Rare Phenomenon
Unusual clinical course
Muhammed Atere1ABCDEF*, Krisha Arora1BCDEF, Urvi Bhavsar1BCDEF, Farhang Ebrahimi2E, Jay M. Nfonoyim3E, Jessie Saverimuttu4EDOI: 10.12659/AJCR.924264
Am J Case Rep 2020; 21:e924264
Abstract
BACKGROUND: The management of patients with end-stage kidney disease can be accomplished with hemodialysis via a surgically created arteriovenous fistula. An arteriovenous fistula has an advantage because of the ability to serve as permanent access for hemodialysis over several months to years; however, it has a disadvantage because of its associated vascular and infectious complications. An infectious complication such as explosive pleuritis, which is usually due to respiratory infections, in the setting of an infected arteriovenous fistula site infection, is extremely rare.
CASE REPORT: A 36-year-old man with a past medical history of IgA nephropathy on hemodialysis with a left forearm arteriovenous fistula presented to the Emergency Department because of left flank pain. Despite no recent history or evidence of a respiratory tract infection, he developed explosive pleuritis within 48 h. The presence of Group A Streptococcus at the arteriovenous fistula site coincided with Streptococcus pyogenes infection. The pleural effusion was drained and he was treated with antibiotics. He recovered and was eventually discharged home.
CONCLUSIONS: Explosive pleuritis, although less frequent, is almost always secondary to respiratory tract infections. An arteriovenous fistula site infection may be the source of infection of an internal organ if no apparent source is identified.
Keywords: Arteriovenous Fistula, Empyema, Pleural Effusion, Pleurisy, Streptococcus pyogenes, Arteriovenous Shunt, Surgical, Kidney Failure, Chronic, Renal Dialysis, Streptococcal Infections, Surgical Wound Infection
Background
Certain diseases cause irreversible damage to the kidneys, and the affected patients can become dialysis-dependent. Hemodialysis can be accomplished by surgically creating an arteriovenous fistula (AVF) in the arm. Infectious and noninfectious complications are more prevalent in patients with hemo-dialysis catheters and grafts than in patients with AVFs [1–4]. For this reason, an AVF is preferred for hemodialysis [1–4]. Nonetheless, an AVF is still prone to noninfectious and infectious problems. Noninfectious complications, which are usually vascular, include ischemic steal syndrome, ischemic monomelic neuropathy, stenosis, thrombosis, fistula hemorrhage, venous hypertension, aneurysms, and pseudoaneurysms [3,5,6]. Infectious complications are secondary to common bacteria like
Our patient developed explosive pleuritis, with the AVF as the likely source of infection. Explosive pleuritis has been described in the literature but it is mainly associated with respiratory tract infections [7,8]. Other etiologies appear to be less frequent. Explosive pleuritis is a term used to describe a rapid accumulation of exudative pleural fluid/empyema, usually within a short time. We report a rare phenomenon in which our patient developed explosive pleuritis, likely from an AVF site infection, despite the absence of recent or concurrent respiratory tract infection or other sources of infection.
Case Report
DAY 0:
A chest x-ray (CXR) done at 0829 h revealed clear lungs (Figure 1). A computed tomography (CT) with contrast of the abdomen and pelvis showed no evidence of acute appendicitis, diverticulitis, or mechanical bowel obstruction, and there was no ascites or evidence of portal hypertension, but both kidneys were atrophic. He had never had a previous chest CT done before the present admission.
DAY 1:
While in the hemodialysis unit, an open wound at the AVF fistula was noticed. The patient denied being aware of a wound at this site. The wound was swabbed and sent for cultures. It was examined and dressed regularly. It eventually grew Streptococcus group A (GAS), likely Streptococcus pyogenes. His WBC increased to 12.5 k/ul and he also became hypotensive. Blood was drawn for cultures and vancomycin and meropenem administrations were initiated. A right internal jugular (IJ) hemodialysis catheter was inserted for dialysis because of the wound at the AVF. He was transferred to the Medical Intensive Care Unit on the same day because of a significant decrease in blood pressure, but it improved after the administration of intravenous fluids. A CXR at 1440 h revealed a moderate left pleural effusion (Figure 2).
DAY 2:
A chest CT at 0912 h showed a very large left pleural effusion with near-complete atelectasis of the left upper lobe and complete atelectasis in the left lower lobe (Figure 3). A CXR at 0930 h demonstrated a near-complete opacification of the left lung with a mediastinal shift to the right (Figure 4). The finding was consistent with a large left pleural effusion with atelectasis in the left lung. A pigtail chest tube was inserted into the left hemithorax. A follow-up CXR at 1451 h showed a left-sided pigtail catheter in situ but with a resolved large left pleural ef-fusion (Figure 5). About 1.3 L of pleural fluid was removed, with fluid analysis showing a cloudy fluid, WBC of 15 762/cumm, red blood cell of 3556/cumm, polynuclear white blood cell of 84%, glucose of 2 mg/dL, total protein of 4.2, and lactate dehydrogenase (LDH) of 9208 u/L. A serum protein was 5.1 g/dl and serum LDH was 150 u/l. Applying Light’s criteria, the pleural fluid was deemed exudative. Clindamycin was added to reduce possible toxin production by GAS.
DAY 7:
A repeat CXR at 0400 h showed a small, likely partially loculated, residual left effusion. The left pigtail catheter was subsequently removed but he continued to have pain at the chest tube insertion site.
DAY 8:
A repeat CXR at 0400 h status-post removal of the left pigtail catheter revealed a re-accumulation of pleural fluid. A video-assisted thoracoscopic surgery (VATS) with pleural drainage and decortication with the insertion of 3 chest tubes (anterior, lateral and posterior) in the left hemithorax were performed.
DAY 10:
A chest CT at 1813 h demonstrated improved left hemithorax and loculated pleural effusion/emphysema, with a small residual area measuring 1.5 cm in thickness and a right-sided pleural effusion (Figure 6).
DAY 12:
The left anterior and lateral chest tubes were removed.
DAY 15:
The left posterior chest tube was removed.
We describe other relevant events during admission. The patient was in a state of sepsis from the first day after hospitalization to the next 2 days, based on persistent intermittent fever spikes, tachycardia, tachypnea, and hypotension requiring intravenous fluids. Fortunately, his vital signs improved during hospitalization. Blood and pleural fluid cultures remained negative throughout the hospitalization. A transthoracic echocardiogram showed no evidence of valvular vegetations. In spite of the AVF infection, he received regular dialysis approximately every other day via a temporary jugular hemodialysis catheter as an access, but he was discharged with a right tunneled permanent catheter. The AVF was revised and repaired. Meropenem (after 12 days of administration) and clindamycin (after 11 days of administration) were switched to ceftriaxone. He received ceftriaxone for a total of 14 days, but he was discharged to continue with vancomycin for a total duration of 6 weeks. He recovered gradually and was discharged home after 27 days of hospitalization.
Discussion
AVFs are generally created surgically in patients who require long-term hemodialysis. It was first performed in the United States in 1965 [9]. Although it has been a groundbreaking procedure, it is not without complications. Such complications may be vascular or non-vascular (infectious). Vascular complications vary from ischemic steal syndrome and ischemic monomelic neuropathy to stenosis and thrombosis [3,5,6]. Other vascular complications include fistulas, hemorrhage, venous hypertension, aneurysms, and pseudoaneurysms [3,5,6]. All the aforementioned drawbacks often require revisions of the AVFs. The other major complication of AVFs is the infectious type, which is the main focus of our case.
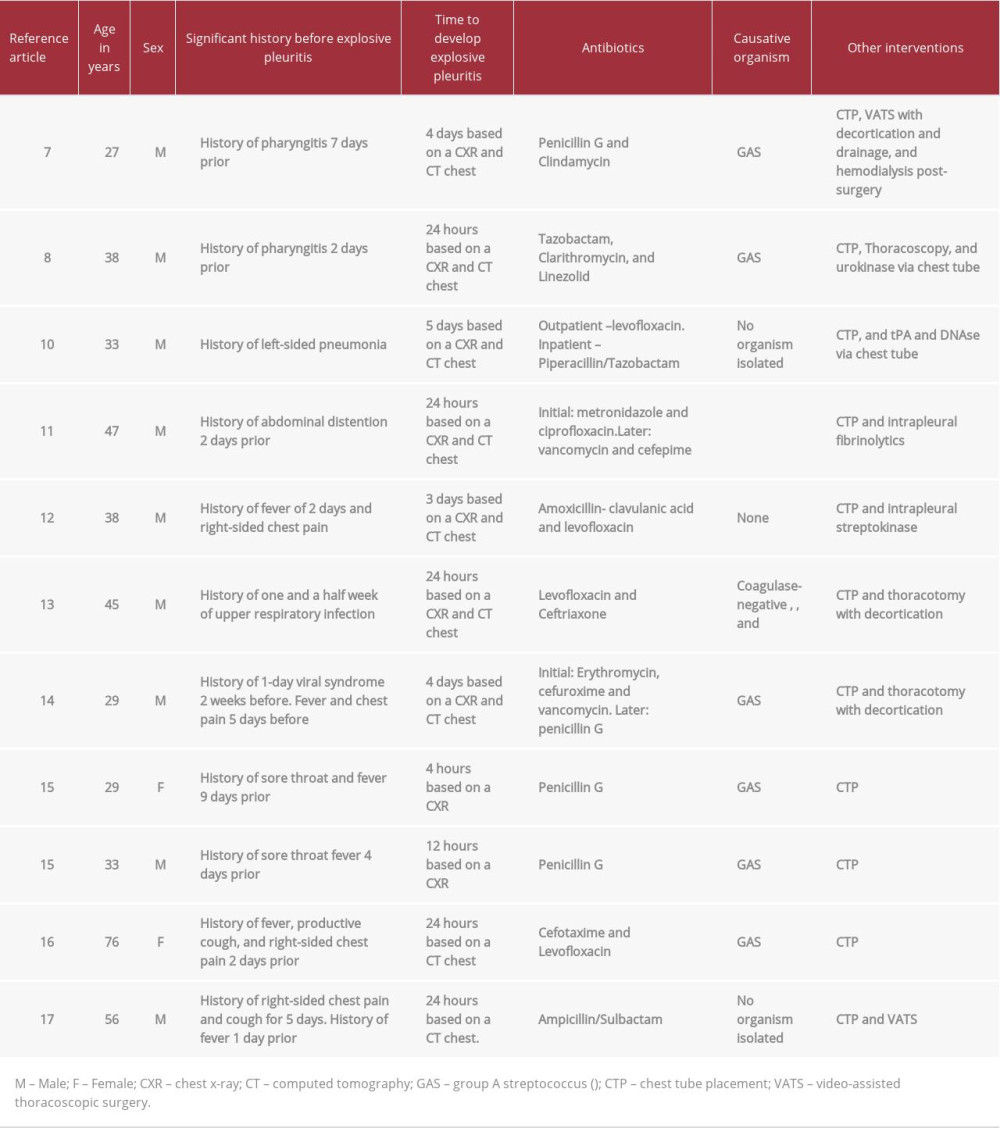
The pathogens identified in the reviewed case reports ranged from GAS, coagulase-negative
Conclusions
Our patient came in initially because of a left-sided abdominal pain. An abdomen CT revealed no obvious pathology. During hospitalization, he developed pyrexia and leukocytosis, and inflammatory markers were elevated. Oozing from his AVF was incidentally discovered and cultures grew GAS. He developed explosive pleuritis within 48 h. The fluid was drained but it reaccumulated and subsequently drained again. Initial antibiotics were vancomycin and meropenem with the addition of clindamycin. Meropenem was discontinued and switched to ceftriaxone. He was discharged on vancomycin to complete a total of 6 weeks of antibiotics. VATS with decortication was performed, the AVF was also regularly dressed and revised, and he was dialyzed via a temporary hemodialysis catheter. The uniqueness of our case is an explosive pleuritis, likely from an infected AVF site, despite negative blood and pleural cultures. It is a rare phenomenon that has rarely been described in the medical literature. We report this case so that physicians should always evaluate AVFs as the potential source of infection of other organs if there are no identifiable risk factors or other apparent sources.
Figures
References:
1.. Ravani P, Gillespie BW, Quinn RR, Temporal risk profile for infectious and noninfectious complications of hemodialysis access: J Am Soc Nephrol, 2013; 24(10); 1668-77
2.. MacRae J, Dipchand C, Oliver M, Arteriovenous access: Infection, neuropathy, and other complications: Can J Kidney Health Dis, 2016; 3; 2054358116669127
3.. Al-Jaishi AA, Liu AR, Lok CE, Complications of the arteriovenous fistula: A systematic review: J Am Soc Nephrol, 2017; 28(6); 1839-50
4.. Ravani P, Quinn R, Oliver M, Examining the association between hemodialysis access type and mortality: The role of access complications: Clin J Am Soc Nephrol, 2017; 12(6); 955-64
5.. Padberg FT, Calligaro KD, Sidawy AN, Complications of arteriovenous hemo-dialysis access: Recognition and management: J Vasc Surg, 2008; 48(5); S55-80
6.. Simon E, Long B, Johnston K, Summers S, A case of brachiocephalic fistula steal and the emergency physician’s approach to hemodialysis arteriovenous fistula complications: J Emerg Med, 2017; 53(1); 66-72
7.. Al-Mashat M, Moudgal V, Hopper JA, Explosive pleurisy related to group A streptococcal infection: A case report and literature review: Pulmonary Research and Respiratory Medicine Open Journal, 2015; 2(3); 109-13
8.. Sharma BD, Kumar A, Nakra V, Explosive pleuritis: Presenting as a life-threatening condition in a young adult: Journal, Indian Academy of Clinical Medicine, 2017; 18(4); 290-93
9.. Konner K, History of vascular access for haemodialysis: Nephrol Dial Transplant, 2005; 20(12); 2629-35
10.. Zoumot Z, Wahla AS, Farha S, Rapidly progressive pleural effusion: Clevel Clin J Med, 2019; 86(1); 21-27
11.. Hatem NA, Matthews K, Foroozesh M, Rapidly progressive parapneumonic effusion: A case of explosive pleuritis: Am J Respir Crit Care Med, 2016; 193; A3316
12.. Kumar S, Sharath Babu NM, Kaushik M, Explosive pleuritis: Online J Health Allied Scs, 2011; 10(4); 12
13.. Sharma JK, Marrie TJ, Explosive pleuritis: Can J Infect Dis, 2001; 12(2); 104-7
14.. Johnson JL, Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician: Chest, 2001; 119; 1266-69
15.. Braman SS, Donat WE, Explosive pleuritis, Manifestation of group A beta-hemolytic streptococcal infection: Am J Med, 1986; 81; 723-26
16.. Fonseca Aizpuru EM, Nuño Mateo FJ, Otero Guerra L, López de Mesa C, [Community acquired pneumonia and explosive-pleuritis]: An Med Interna, 2005; 22(8); 401-2 [in Spanish]
17.. Ishikawa O, Gabra NI, Mahmoud O, Rare and rapid: A case of explosive pleuritis: Chest, 2019; 156(4); A1809-10
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250