15 July 2020: Articles 
Tigecycline-Induced Severe Hypoglycemia in a Non-Diabetic Individual: A Case Report and Brief Review of Tigecycline-Induced Severe Hypoglycemia
Unusual or unexpected effect of treatment, Unexpected drug reaction
Avik Ray1ABCDEF*, Swati Sharma1ABDEF, Shubham Atal1DEF, Balakrishnan Sadasivam1DEF, Ratinder Jhaj1EFDOI: 10.12659/AJCR.924556
Am J Case Rep 2020; 21:e924556
Abstract
BACKGROUND: Tigecycline is a broad-spectrum antibiotic belonging to the glycylcycline class. Hypoglycemia is a rare adverse effect associated with its use. We report a case of multiple episodes of severe hypoglycemia in a non-diabetic patient on treatment with tigecycline for cellulitis following snake bite.
CASE REPORT: A 45-years-old male was admitted with cellulitis of left leg due to snake bite with sepsis, acute kidney injury, and disseminated intravascular coagulation. A transtracheal aspirate culture showed infection with Klebsiella pneumoniae, and parenteral tigecycline was started based on the drug-sensitivity testing results. Multiple severe hypoglycemic episodes occurred, with persistently low blood glucose levels in the subsequent days. Tigecycline was stopped at the physician’s discretion due to completion of the recommended course of treatment. The blood glucose levels continued to remain either below or on the lower end of the normal range before it started rising gradually. Tigecycline was again started based on another drug-sensitivity testing. A total of 4 episodes of hypoglycemia occurred over the next 2 days, and tigecycline was stopped prematurely. The patient’s condition gradually improved with no more hypoglycemic episodes, and he was finally discharged.
CONCLUSIONS: We report a non-diabetic patient of cellulitis following snake bite who suffered from tigecycline-induced severe hypoglycemic episodes that persisted for a prolonged period of time. After a thorough search of the published literature, we could not find such a case of severe and sustained hypoglycemia due to tigecycline in an individual without diabetes and not on any hypoglycemic agent.
Keywords: Diabetes Mellitus, Hypoglycemia, Intensive Care Units, Anti-Bacterial Agents, Cellulitis, Klebsiella Infections, Snake Bites, Tigecycline
Background
Tigecycline is a broad-spectrum antibiotic belonging to the glycylcycline class and with an activity spectrum broader than its congeners, the tetracyclines. It is a derivative of minocycline structurally and is effective even against bacteria that have acquired resistance to tetracyclines. This is due to evasion of the 2 primary resistance mechanisms – efflux pumps and ribosomal protection protein [1]. Tigecycline has activity against resistant strains of gram-positive bacteria like methicillin-resistant
Owing to the broad range of activity, tigecycline is indicated for treatment of complicated dermatological and intra-abdominal infections along with community-acquired bacterial pneumonia, for which it can be used as a single agent [4]. It is administered intravenously at a 100-mg loading dose and at a maintenance dose of 50 mg every 12 h in an adult patient. The maintenance dose is reduced to 25 mg every 12 h for individuals with hepatic impairment [4].
Adverse effects commonly reported with use of tigecycline are nausea, vomiting, and diarrhea [4]. Hypoglycemia is a rare adverse effect, with only 39 such events reported in the Vigiaccess database to date [5]. We report a case of severe hypoglycemia in a non-diabetic patient of cellulitis following snake bite, on treatment with tigecycline in the intensive care unit (ICU) of a tertiary care hospital.
Case Report
A 45-year-old male farmer presented to the Emergency Room (ER) of All India Institute of Medical Sciences (AIIMS) Bhopal on 6 August 2019 with shortness of breath, altered sensorium, and inability to speak, along with weakness of bilateral lower limbs. There was a history of suspected snake (species unknown) bite on the left foot 2 days before. There was swelling of the left leg, with anasarca. According to family members who brought him to the ER, the snake had bitten him while he was working in a field, after which he felt pain at the site of the bite. He had described the snake as “something long” to them. He had been treated at a peripheral hospital for the previous 2 days. Anti-snake venom (ASV) was mentioned in the discharge summary of the hospital, but according to the family members, it was not given. He had been administered some medications, for which records were not available. The patient was non-diabetic and without any history of hypertension, tuberculosis, or any other chronic disease. There were no episodes of melaena or any bleeding manifestations. There was a history of smoking and intake of alcohol.
The patient was preliminarily examined at the ER. He was minimally conscious with reduced alertness, non-cooperative, and disoriented. Blood pressure, as measured by a digital electronic sphygmomanometer, was 106/60 mmHg with a cardiac rate of 102 beats per minute. The respiratory rate, as recorded on arrival, was 20 breaths per minute, body temperature was 37.2°C, and SpO2 was 91%. Bilateral crepitations could be heard over the chest on auscultation. There was pitting edema over the left foot, which was non-tender in nature, with raised temperature. Non-distinct impressions of two rows of teeth without any fang marks could be seen over the swelling, indicating a possible non-venomous snake bite.
Laboratory investigations showed that hemoglobin level was 15 g/dl, total leukocyte count was 24.37×109/L (neutrophils: 89%, lymphocytes: 6%), platelet count was 19,000 per cubic millimeter, blood urea was 122 mg/dl, serum creatinine was 5.01 mg/dl, with serum sodium and potassium concentrations of 117 mEq/L and 5.1 mEq/L, respectively. A lower-limb color Doppler examination did not show any signs of deep vein thrombosis. An ultrasonography (USG) of the whole abdomen showed bilateral mildly raised renal cortical echo-genicity. Coagulation profile was normal with PT of 12.7 s (Control: 13.2 s) and INR of 1.03. Serum procalcitonin level was 0.27 ng/ml. A 2D echocardiography revealed normal left ventricular function while lung USG showed bilateral multiple B lines, indicating interstitial pulmonary edema.
Following intubation, the patient was transferred to the ICU with a provisional diagnosis of snake bite with cellulitis of the left leg with sepsis, acute kidney injury (AKI), and disseminated intravascular coagulation (DIC). Dual-antibiotic treatment was started with intravenous (IV) piperacillin plus tazobactam (4.5 g every 8 h) and IV clindamycin (600 mg every 8 h). Magnesium sulphate dressing was done twice a day at the site of swelling. Six units of random donor platelets (RDP) were transfused. The patient was put on continuous renal replacement therapy (CRRT) the following day. Other medications included IV metoclopramide (10 mg every 8 h), oral calcium polystyrene sulfonate (every 6 h), IV glucose-insulin drip (300 ml every 8 h), oral pantoprazole (40 mg once daily), IV vitamin K (10 mg once daily) and IV magnesium sulphate (1 g every 12 h). Oral conjugated estrogen (0.625 mg every 8 h) was also added due to uremic bleeding.
Over the next couple of days, the condition of the patient worsened, with no signs of reduction in the swelling. The body temperature varied between 37.8°C and 38.9°C, with persistence of bilateral chest crepitations. Blood glucose levels were in the normal range (70–100 mg/dl). On day 7, the total leukocyte count was 31.65×109/L (neutrophil: 91%, lymphocyte: 7%). On day 8, the results of transtracheal aspirate culture showed infection with
On day 10, the patient had a hypoglycemic episode immediately after administration of the morning dose of tigecycline (Figure 2), manifesting with severe palpitations, tremors, and sweating. His blood glucose level was 47 mg/dl, as measured using a glucometer. He was given 100 ml of 25% dextrose infusion, after which symptoms were relieved promptly and the blood glucose increased to 83 mg/dl. Another hypoglycemic episode happened the next day after tigecycline administration (day 11); the blood glucose level was 49 mg/dl and he was hyperventilating with profuse sweating and palpitations. It was managed again with 100 ml of 25% dextrose infusion, and the blood glucose level rose to 86 mg/dl. Similar episodes happened later on the same day and on day 13 followed by same management in both instances.
Tigecycline was stopped on day 15 (Figure 3) at the treating physician’s discretion because the course of antibiotic treatment was completed. IV meropenem (500 mg every 12 h), IV clindamycin (600 mg every 8 h), and IV polymyxin B (10,000 units every 12 h) were added in place of tigecycline, but the other medications remained unchanged. Although there were no further hypoglycemic episodes, the blood glucose levels continued to be either below or on the lower end of the normal range. Starting from day 17, the blood glucose levels started rising gradually and were well maintained within and slightly above the normal range (Figure 4).
The condition of the patient improved rapidly with gradual reduction of the swelling. CRRT was stopped on day 19 after serum creatinine and urea levels and electrolyte levels remained consistently in the normal ranges. However, signs of sepsis reappeared on day 27. A transtracheal aspirate was sent for culture and the results came back on day 29, revealing infection with Enterobacter spp. (>105 colony count) sensitive to doxycycline, minocycline, tetracycline, and tigecycline. Meropenem and polymyxin B were replaced with IV tigecycline (loading dose of 100 mg; subsequently 50 mg every 12 h) on the same day.
Three successive episodes of hypoglycemic attacks accompanied by similar symptoms of sweating and palpitations took place the next day and the day after (Figures 5, 6), with the blood glucose level falling as low as 25 mg/dl. We administered 50 ml of 50% dextrose infusion each time to restore the glucose levels back to normal. After evaluating the patient’s clinical condition and ongoing medications, tigecycline was considered as the probable cause for the severe hypoglycemic episodes and it was discontinued on day 31. Oral minocycline (100 mg every 12 h) and IV cefoperazone plus sulbactam (1.5 g every 6 h) were added in place of tigecycline along with other ongoing medicines.
There were no further hypoglycemic episodes, although the blood glucose levels stayed on the lower end of normal range or slightly below it until the following day. The condition of the patient improved gradually, with slow reduction of swelling and reversal of sepsis. Finally, the patient was discharged from the ICU on day 38 and was transferred to the general medicine ward for further evaluation and management.
Discussion
Hypoglycemia is a rare adverse effect associated with tigecycline therapy. However, episodes of hypoglycemia with tigecycline, as reported to date, have been reported in patients with diabetes who were already on one or more glucose-lowering agents [6–8]. An epidemiologic surveillance study of hypoglycemia associated with antibiotic usage using the US Food and Drug Administration Adverse Event Reporting System (FAERS) revealed the association of tigecycline with hypoglycemia, even if not taken with oral hypoglycemic agents such as sulfonylureas and meglitinide [9]. Our patient did not have diabetes and was not taking any hypoglycemic agent. However, the hypoglycemia was severe and persisted for an extended period of time in the absence of any glucose-lowering treatment. Such a case has not been reported before.
Tigecycline is not an extensively metabolized drug; only a small fraction of it undergoes biotransformation via glucuronidation (not more than 10% of the dose) [4]. The elimination half-life of multiple doses of tigecycline (50 mg every 12 h) is approximately 42 h; 59% of the dose is excreted via biliary/fecal route while 33% is excreted in urine, and 22% is removed in unchanged form [4]. Based on the results of a single-dose study of tigecycline in healthy subjects, patients with reduced creatinine clearance, and patients with end-stage renal disease (ESRD), dose adjustments are not required in patients with AKI or for those having ESRD and who are on CRRT [7]. Hence, we did not adjust the dose for our patient.
Blood glucose levels, which were normal earlier, fell after administration of tigecycline, remained low throughout the entire duration of tigecycline administration, and rose slowly after the discontinuation of the antibiotic for the first time. Levels fell sharply again after tigecycline was re-introduced and improved gradually after it was stopped for the second time, clearly indicating a temporal association and suggesting a causal relationship of tigecycline with hypoglycemia. Hypoglycemia persisted with episodic dips after tigecycline administration throughout the entire duration of the treatment in two separate courses with the drug. There was a positive de-challenge in between the two course of treatment and a positive re-challenge, within the second course with daily administration of tigecycline, leading to episodes of hypoglycemia, followed by a positive de-challenge.
None of the other medications co-administered with tigecycline during both courses of treatment are known to cause hypoglycemia. The first episode of hypoglycemia, which was severe in intensity, happened 35 h after the first dose of tigecycline, consistent with a previous case [6]. Furthermore, the hypoglycemia persisted for approximately 40 h after the last dose of tigecycline, in both instances after discontinuation, consistent with the elimination pharmacokinetics of tigecycline [4].
All these factors strongly suggest that tigecycline caused the hypoglycemia. The Naranjo algorithm for adverse drug reaction causality assessment [10] revealed a “definite” relationship (total score: 9) between hypoglycemia and tigecycline (Table 1). We reported this case to the Adverse Drug Reaction (ADR) Monitoring Center of the institute (AIIMS Bhopal). Based on the published evidences to date, the cause of hypoglycemia associated with tigecycline can be hypothesized due to increased insulin release from beta islet cells of the pancreas, inhibition of alpha-glucosidase and alpha-amylase enzymes, and/or increased insulin sensitivity.
Conclusions
We report a non-diabetic patient with cellulitis following snake bite who experienced tigecycline-induced severe hypoglycemic episodes. The hypoglycemia continued for long time, even after stoppage of tigecycline, which is consistent with its pharmacokinetics. The Naranjo algorithm gave a score of 9, indicating a “definite” relationship between administration of tigecycline and hypoglycemia. Hypoglycemia is a serious adverse event and prompt corrective measures need to be taken to prevent it from being fatal. Further studies and investigations are required to understand the underlying mechanism of hypoglycemia caused by tigecycline. To the best of our knowledge, this is the first reported case of tigecycline-induced severe and sustained hypoglycemia in a non-diabetic patient who was not on any hypoglycemic agent.
Figures
References:
1.. Bauer G, Berens C, Projan SJ, Hillen W, Comparison of tetracycline and tigecycline binding to ribosomes mapped by dimethylsulphate and drug-directed Fe2+ cleavage of 16S rRNA: J Antimicrob Chemother, 2004; 53(4); 592-99
2.. Zhanel GG, Homenuik K, Nichol K, A comparative review with the tetracyclines: Drugs, 2004; 64(1); 63-88
3.. Nathwani D, Tigecycline: Clinical evidence and formulary positioning: Int J Antimicrob Agents, 2005; 25(3); 185-92
4.. : Tygacil (tigecycline) for injection [package insert], 2005, Philadelphia, PA, Wyeth Pharmaceuticals Inc.
5.. VigiAccess™. Available from: http://www.vigiaccess.org/
6.. Chen Y, Li L, Zhang N, Li H, Tigecycline-induced sustained severe hypoglycemia: A case report: BMC Pharmacol Toxicol, 2019; 20(1); 50
7.. Lauf L, Ozsvár Z, Mitha I, Phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis: Diagn Microbiol Infect Dis, 2014; 78(4); 469-80
8.. Shah B, Kaushik S, Tigecycline induced hypoglycemia: A case report: Res J Pharm Biol Chem Sci, 2014; 5(4); 1021-23
9.. Kennedy KE, Teng C, Patek TM, Frei CR, Hypoglycemia associated with antibiotics alone and in combination with sulfonylureas and meglitinides: An epidemiologic surveillance study of the FDA Adverse Event Reporting System (FAERS): Drug Saf, 2020; 43(4); 363-69
10.. Naranjo CA, Busto U, Sellers EM, A method for estimating the probability of adverse drug reactions: Clin Pharmacol Ther, 1981; 30(2); 239-45
Figures
Tables
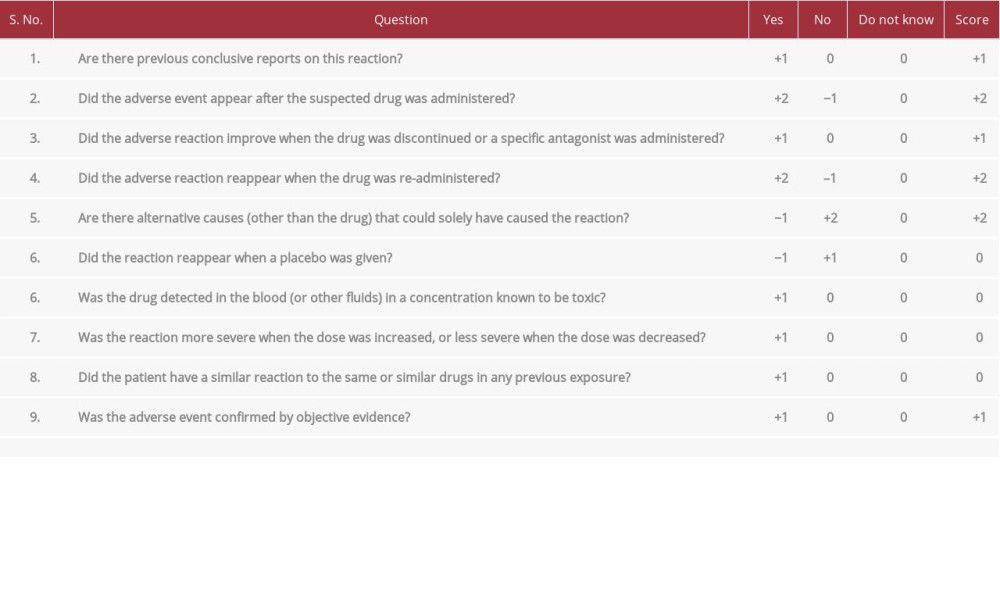 Table 1.. The patient’s score for the Naranjo algorithm for adverse drug reaction causality assessment. Total score categories are defined as follows: Adverse Drug Reaction (ADR) is: definite ≥9; probable 5–8; possible 1–4; doubtful 0.
Table 1.. The patient’s score for the Naranjo algorithm for adverse drug reaction causality assessment. Total score categories are defined as follows: Adverse Drug Reaction (ADR) is: definite ≥9; probable 5–8; possible 1–4; doubtful 0. Table 1.. The patient’s score for the Naranjo algorithm for adverse drug reaction causality assessment. Total score categories are defined as follows: Adverse Drug Reaction (ADR) is: definite ≥9; probable 5–8; possible 1–4; doubtful 0.
Table 1.. The patient’s score for the Naranjo algorithm for adverse drug reaction causality assessment. Total score categories are defined as follows: Adverse Drug Reaction (ADR) is: definite ≥9; probable 5–8; possible 1–4; doubtful 0. In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








