13 October 2020: Articles 
An Autopsy Case of TAFRO Syndrome with Type II Respiratory Failure
Rare disease
Mikio Wada1ABCDEF*, Akihiro Nagata2BCDE, Atsushi Kawashima3ABCDEF, Keizo Kagawa3DEFDOI: 10.12659/AJCR.926721
Am J Case Rep 2020; 21:e926721
Abstract
BACKGROUND: TAFRO syndrome (thrombocytopenia, anasarca, fever, myelofibrosis, renal dysfunction, and organomegaly) is a systemic inflammatory disorder. The histological features of TAFRO syndrome are not fully understood and few autopsy cases have been reported.
CASE REPORT: A 66-year-old man with type II respiratory failure was diagnosed with TAFRO syndrome. He was initially treated with tocilizumab. Although some improvements were observed, his condition worsened, and the medication was switched to rituximab. His condition remained steady for 1 year with intermittent artificial ventilation. However, he died due to exacerbation of respiratory failure about 20 months after diagnosis. An autopsy revealed mucous fluid retention in the spaces between the axis cylinder and the myelin sheath of peripheral nerves and among the peripheral nerves, suggesting that this retention contributed to neurodegeneration with demyelination. Skeletal muscles, including respiratory muscles, were highly atrophic, which could have led to type II respiratory failure.
CONCLUSIONS: Fluid accumulation other than pleural effusion and ascites could occur in intra-organs at a cellular level.
Keywords: Edema, Giant Lymph Node Hyperplasia, Nerve Degeneration, Respiratory Insufficiency, Autopsy, rituximab
Background
TAFRO syndrome is a systemic inflammatory disorder characterized by thrombocytopenia, anasarca, fever, myelofibrosis, renal dysfunction, and organomegaly [1]. The first 3 cases of TAFRO syndrome were reported in 2010 [2], and Masaki et al. proposed diagnostic criteria for TAFRO syndrome and a disease severity classification system in 2015 [3], which were further updated in 2019 [4]. However, the histological features of TAFRO syndrome are not fully understood. Indeed, few studies have reported histological findings other than kidney and lymph node involvement. Moreover, few autopsy cases have been published. Here, we report an autopsy case of TAFRO syndrome complicated with type II respiratory failure due to peripheral nerve disorder. This is a follow-up report of our previous study [5].
Case Report
A 66-year-old man with complaints of dyspnea and general fatigue was admitted to our hospital. He had experienced pitting edema in both legs 4 years prior to admission, with no other symptoms. Pleural effusion and ascites were documented at a different hospital. Biopsy of enlarged axillary lymph nodes and bone marrow were performed at another hospital 1 year prior to the present admission, but no definitive diagnosis was made. Upon worsening of his general condition, he was referred and admitted to our hospital.
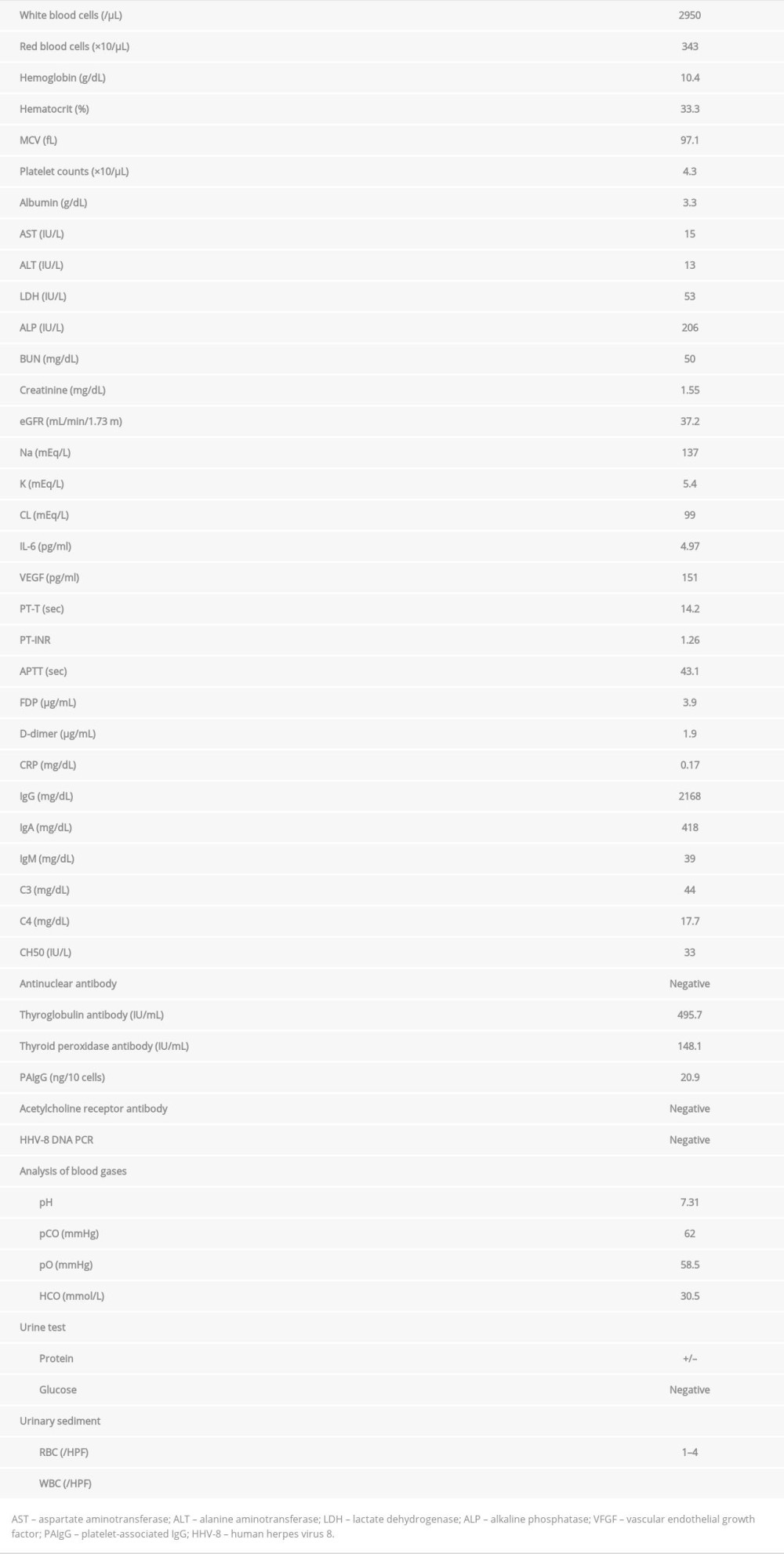
He was conscious and oriented on admission. His body temperature was 37.7°C, blood pressure 140/80 mmHg, pulse rate 50/min, respiratory rate 20/min, and oxygen saturation 92% without supplemental oxygen administration. After admission, we re-evaluated pathological tissues obtained from the previous medical institution. Left axillary lymph nodes showed interfollicular expansion, atrophic germinal centers, and arborized blood vessels (Figure 1A, 1B). We also noted infiltration of small lymphocytes and plasma cells, and confirmed the finding of non-monoclonality of infiltrating plasma cells [5]. Increased megakaryocytes and reticular fibers were observed in the bone marrow biopsy (Figure 1C, 1D). We made the diagnosis of TAFRO syndrome based on patient history, laboratory data (Table 1), computed tomography (CT) showing bilateral pleural effusion and moderate lymphadenopathy, results of pathological evaluations, and other findings reported previously [5]. Results of blood gas analysis and a respiratory function test revealed type II respiratory insufficiency and restrictive impairment (%VC: 30.1%, FEV1.0%: 93.5%).
Treatment with tocilizumab was initiated, achieving gradual improvements in general condition as well as laboratory findings such as thrombocytopenia and renal dysfunction; however, respiratory function did not sufficiently improve. He was discharged on day 35. Administration of tocilizumab once every other week was continued in an outpatient setting, every 3 weeks from day 148, and once every 4 weeks beginning on day 211 (Figure 2). Then, the patient was re-admitted on day 267 due to exacerbation of thrombocytopenia and renal dysfunction. Suspecting that TAFRO syndrome had worsened, the frequency of tocilizumab administration was increased back to bi-weekly administration. His overall condition improved and he was discharged on day 283. However, general fatigue progressed and a worsened respiratory state was confirmed on day 286, resulting in re-hospitalization. Gradual worsening of ability to perform activities of daily living (ADL) was also noted. Despite pulse therapy with methylprednisolone (1000 mg/day for 3 days) on day 288, his respiratory condition worsened further, and he was placed on an artificial respirator. At this point, we switched to rituximab 500 mg (375 mg/m2), and laboratory values improved and respiratory status also improved somewhat. A tracheotomy was performed on day 314, after which intermittent ventilation was needed. He was discharged on day 442, since his general condition (other than respiratory status) was satisfactory. While previous studies reported that respiratory failure was related to myasthenia gravis in some cases of Castleman’s disease [6,7], anti-acetylcholine receptor antibodies were negative in our patient. A previous nerve conduction study of our patient [5] revealed axonal involvement and demyelination changes, leading us to suspect peripheral nerve disorder.
Despite respiratory discomfort and worsened ADL, the patient’s overall condition was generally stable for several months with rituximab treatment every 4 weeks. However, he was re-admitted on day 584 due to worsening of general fatigue and appetite loss. Mild fluid accumulation without consolidation in the right chest was observed on CT. Laboratory findings showed slight CRP elevation and worsened renal function. Thrombocytopenia was observed 3 days after re-admission. Cultures of sputum and urine were negative. Suspecting that TAFRO syndrome had worsened, rituximab was administered earlier than the scheduled (roughly 2 weeks after the previous administration) but was not effective. The patient and his family wished no further treatment. His general condition gradually worsened and he died on day 611.
An autopsy revealed pleural effusions (left: 40 mL, hemorrhagic due to bronchopneumonia; right: 1000 mL, serosity) and as-cites (1500 mL, serosity). Parenchyma of the left lung was increased in weight (left 675 g, right 430 g) and had marked bronchopneumonia, but the condition did not appear critical enough to have cause respiratory failure. We then sought to determine the cause of type II respiratory failure. Peripheral nerves were degenerated (neurodegeneration) with demyelinating and edematous changes. Spaces around the peripheral nerves of the skeletal muscle (e.g., breathing muscle) were positive for Alcian Blue staining and negative for hyaluronidase staining (Figure 3A, 3B). Retention of mucus was suspected between the axis cylinder and myelin sheath, but no lymphocyte accumulation was observed in those areas. Retention of mucus among peripheral nerves was also suspected. Little staining was observed with Klüver-Barrera staining (Figure 3C, 3D). Overall, highly atrophic skeletal muscles, including respiratory muscles (Figure 3E), atrophic lymph nodes, notable high endothelial venules (Figure 4A, 4B), atrophic white pulps of the spleen, and notable red pulps were observed. A membranoproliferative glomerulonephritis-like appearance was noted across the kidney. Lobular formation with mesangial proliferation was found in almost all glomeruli, and many glomeruli showed crescent formation (Figure 4C, 4D).
Discussion
TAFRO syndrome was defined at a Japanese consensus meeting in 2012 [1], and its diagnostic criteria were first proposed in 2016 and updated in 2019 [4]. Our patient fit all major (anasarca, thrombocytopenia, and systemic inflammation) and minor categories. In terms of disease severity, his condition was slightly severe (grade 3) with 3 points for anasarca, 2 points for thrombocytopenia, and 1 point each for fever and renal insufficiency.
Renal dysfunction associated with TAFRO syndrome varies in severity, with some patients requiring hemodialysis [8–11]. In the present case, while renal function worsened as the disease progressed, treatment with tocilizumab and rituximab achieved some improvement. Histological findings on autopsy revealed a membranoproliferative glomerulonephritis-like appearance, which has also been reported in previous cases of TAFRO syndrome [9,11,12].
The patient had type II respiratory failure. A nerve conduction study confirmed that he had peripheral nerve disorder with axonal involvement and demyelinating changes. In autopsy, histological findings revealed that spaces between the axis cylinder and myelin sheath of peripheral nerves in skeletal muscles were positive for Alcian Blue staining and negative for hyaluronidase staining, and retention of mucous fluid was suspected. This and the Klüver-Barrera staining of peripheral nerves confirmed neurodegeneration with demyelination of peripheral nerves, which were consistent with results of the nerve conduction study. Overall, skeletal muscles, including respiratory muscles, were highly atrophic. This could have led to his type II respiratory failure, which worsened and was eventually fatal. Since type II respiratory failure developed early on, we speculate that the nerve disorder could have also developed from an early stage of the disease.
A possible differential diagnosis was POEMS (Polyneuropathy, Organomegaly, Endocrinopathy, M-protein, Skin changes) syndrome [13]. Restrictive lung disease with neuromuscular weakness has been reported in some cases of POEMS syndrome [14]. However, as the patient did not have monoclonality for plasma cells or skin changes, the patient was unlikely to have POEMS syndrome. Both TAFRO syndrome and POEMS syndrome resemble Castleman’s disease in some respects. For instance, POEMS syndrome includes peripheral neuropathy in the disease definition. Thus, these diseases might be related in the context of neurodegeneration.
While fluid accumulation, such as pleural effusion and ascites, is one of the characteristics of TAFRO syndrome, few reports have described fluid retention in other intra-organs. In a previously reported case of TAFRO syndrome, the patient developed chronic and asymmetric edema of the optic disc and serous retinal detachment [15]. In our patient, mucous fluid retention was observed in the spaces between the axis cylinder and the myelin sheath of peripheral nerves and among the peripheral nerves. This may suggest the existence of a subpopulation of TAFRO syndrome cases in which fluid accumulation is not limited to pleural effusion and ascites, but also occurs in other intra-organs on a cellular level. However, while tocilizumab treatment did not have a sufficient effect on some patients [16,17], treatment of our case led to a modest improvement of respiratory failure and peripheral nerve disorder. Thus, we cannot rule out the possibility that another disease was involved in the retention of mucous fluid and neurodegeneration.
Conclusions
We described an autopsy case of TAFRO syndrome complicated by type II respiratory failure with atrophic changes in respiratory muscles due to neurodegeneration. Histological findings revealed mucous fluid retention around the peripheral nerves in skeletal muscles, and we speculate this could have contributed to neurodegeneration with demyelination. Further accumulation of cases will be necessary to determine the association of TAFRO syndrome with peripheral nerve disorder and fluid retention.
Figures
References:
1.. Kawabata H, Takai K, Kojima M, Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: A status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012): J Clin Exp Hematop, 2013; 53; 57-61
2.. Takai K, Nikkuni K, Shibuya H, Hashidate H, [Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly]: Rinsho Ketsueki, 2010; 51; 320-25 [in Japanese]
3.. Masaki Y, Kawabata H, Takai K, Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version: Int J Hematol, 2016; 103; 686-92
4.. Masaki Y, Kawabata H, Takai K, 2019 Updated diagnostic criteria and disease severity classification for TAFRO syndrome: Int J Hematol, 2020; 111; 155-58
5.. Aoki T, Wada M, Kawashima A, Tocilizumab-resistant TAFRO syndrome complicated by type II respiratory failure: Intern Med, 2017; 56; 3249-54
6.. Ishikawa K, Kato T, Aragaki M, A case of Castleman’s disease with myasthenia gravis: Ann Thorac Cardiovasc Surg, 2014; 20(Suppl.); 585-88
7.. Wang S, Chen SW, Cai SL, Jin BY, A case report of retroperitoneal pararenal Castleman’s disease associated with myasthenia gravis: World J Surg Oncol, 2014; 12; 331
8.. Tsurumi H, Fujigaki Y, Yamamoto T, Remission of refractory ascites and discontinuation of hemodialysis after additional rituximab to long-term glucocorticoid therapy in a patient with TAFRO syndrome: Intern Med, 2018; 57; 1433-38
9.. Tanaka M, Tsujimoto H, Yamamoto K, Clinicopathological features of progressive renal involvement in TAFRO syndrome: A case report and literature review: Medicine (Baltimore), 2017; 96; e8216
10.. Kawabata H, Kotani S, Matsumura Y, Successful treatment of a patient with multicentric Castleman’s disease who presented with thrombocytopenia, ascites, renal failure and myelofibrosis using tocilizumab, an anti-interleukin-6 receptor antibody: Intern Med, 2013; 52; 1503-7
11.. Hashimoto K, Sano T, Honma Y, An autopsy case of TAFRO syndrome with membranoproliferative glomerulonephritis-like lesions: CEN Case Rep, 2019; 8; 48-54
12.. Furuto Y, Hashimoto H, Horiuti H, Shibuya Y, Membranoproliferative glomerulonephritis-like findings for TAFRO syndrome, associated with an anterior mediastinal tumor: A case report: Medicine (Baltimore), 2018; 97; e11057
13.. Brown R, Ginsberg L, POEMS syndrome: Clinical update: J Neurol, 2019; 266; 268-77
14.. Allam JS, Kennedy CC, Aksamit TR, Dispenzieri A, Pulmonary manifestations in patients with POEMS syndrome: A retrospective review of 137 patients: Chest, 2008; 133; 969-74
15.. Ortiz A, Cardenas P, Peralta M, Neuro-ophthalmological findings in TAFRO syndrome in a patient from South America, a variant of multicentric Castleman’s disease: Int Ophthalmol, 2018; 38; 1641-46
16.. Yamaga Y, Tokuyama K, Kato T, Successful treatment with Cyclosporin A in tocilizumab-resistant TAFRO syndrome: Intern Med, 2016; 55; 185-90
17.. Tatekawa S, Umemura K, Fukuyama R, Thalidomide for tocilizumab-resistant ascites with TAFRO syndrome: Clin Case Rep, 2015; 3; 472-78
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250