20 December 2021: Articles 
Left Atrial Septal Pouch and Acute Thromboembolic Ischemia of the Upper Limb
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Rare coexistence of disease or pathology
Esmeralci Ferreira1AEF*, Dinaldo Cavalcanti de OliveiraDOI: 10.12659/AJCR.932582
Am J Case Rep 2021; 22:e932582
Abstract
BACKGROUND: A left atrial septal pouch (LASP) was first described in 2010 as a new anatomical entity with potential for embolic events. The prevalences of left, right, and double septal pouches are 40.8%, 5.1%, and 3.7%, respectively. There is a concern about the risk of embolic events due to formation of thrombi in a LASP (especially stroke).
CASE REPORT: A 60-year-old man presented with sudden onset of right arm pain associated with sweating and neck pain radiating to his left upper extremity. On physical examination, his right arm was cyanotic and he had pain, paresthesia, and no radial pulse. The patient was diagnosed with acute arterial occlusion of his right upper extremity. An arterial embolectomy was performed with a Fogarty catheter at the level of the brachial artery, which resulted in immediate reperfusion. The patient had an embolic event and after efforts to identify the possible etiology, only an LASP was found. Therefore, we hypothesized that he experienced an embolic event in which a thrombus had formed at the site of the LASP.
CONCLUSIONS: The present case report is designed to raise awareness of the thrombogenic potential of LASP and the possibility of an embolic event to the upper limb of patients with it. LASP can be the source of a thrombus in a patient with a non-stroke embolic event.
Keywords: Atrial Septal Defect 1, Stroke, thromboembolism, Arm, Atrial Appendage, Heart Septal Defects, Atrial, Humans, Ischemia, Male, Thrombosis
Background
A septal pouch (SP) was first described in 2010 as a new anatomical entity with potential for embolic events [1]. It is a kangaroo pouch-like structure in the interatrial septum that is shaped like a cone or cylinder with smaller diverticula originating from the main body. The prevalences of left, right, and double SP are 40.8%, 5.1%, and 3.7%, respectively [2,3].
In patients with a left atrial septal pouch (LASP), there is concern about the risk of embolic events – especially stroke – because of the potential for formation of thrombi in the structure [4]. In the present case report, we describe a patient with a LASP who had an embolic event in his upper limb. This kind of embolization is a rare clinical event.
Case Report
A 60-year-old man presented with sudden onset of right arm pain associated with sweating and neck pain radiating to his left upper extremity. He reported a history of hypertension, drinking, and smoking but did not use illicit drugs nor did he have a family history of heart disease. The patient was taking an angiotensin-converting enzyme (ACE) inhibitor, beta-blockers, and statins.
Physical examination revealed cyanosis in the patient’s right arm, pain, and paresthesia, and no radial pulse. He was diagnosed with acute arterial occlusion of the right upper extremity. An arterial embolectomy was performed using a Fogarty catheter at the level of the brachial artery, which resulted in immediate reperfusion of the affected extremity.
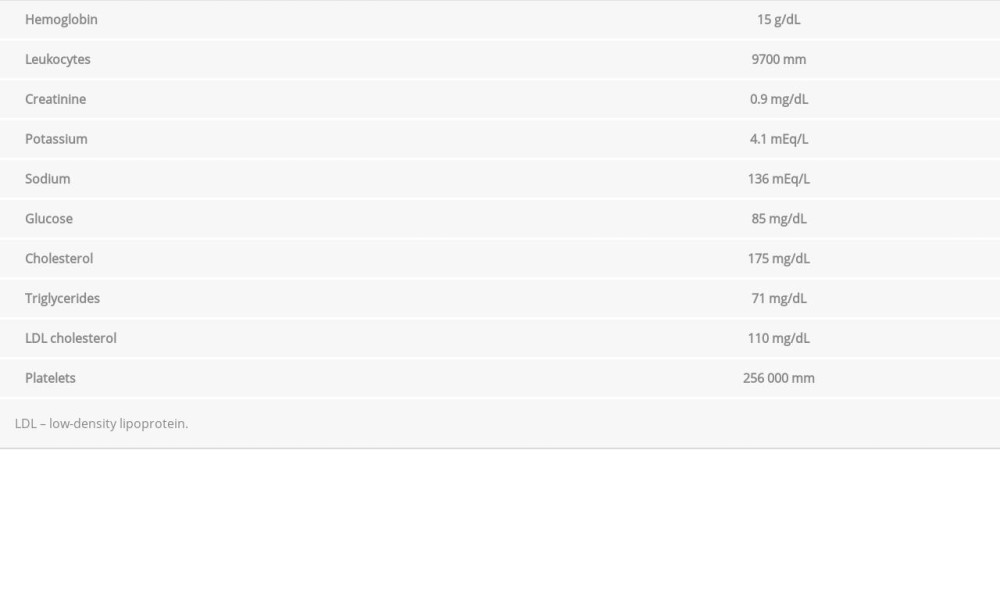
Tables 1 and 2 show the results of the patient’s laboratory tests, all of which were normal. Doppler ultrasound showed no stenoses in his carotid and vertebral arteries. Testing for antithrombin III, protein S, protein C, factor VIII, factor V Leiden, prothrombin gene mutation, lupus anticoagulant, and B2 glycoprotein revealed no abnormalities. A 48-hour Holter monitor showed that the patient had no arrhythmia. Doppler ultrasonography of his upper extremities revealed a thrombosis in the distal third of his ulnar artery.
Computed tomography angiography of his right subclavian artery (Figure 1) and right upper extremity showed that the arteries were of normal diameter. However, the patient’s ulnar artery was thin, with faint irregular opacification in its distal third, corresponding to an area of thrombosis. A transesophageal echo-cardiogram (TEE) showed a left ventricular ejection fraction of 79.3%, normal-sized cavities, and normal wall thickness. A blind-ending saccular structure that measured 1.1×0.3×0.3 cm was detected in the patient’s left atrium (Figure 2). There were no abnormalities in his cardiac valves and there was no intra-cardiac thrombus. His diastolic function was normal. Because the anatomical finding was at the junction of the septum primum and septum secundum, the patient was diagnosed with a LASP without thrombus. He did not have patent foramen ovale (PFO) or atrial septal defect.
On discharge, the patient was asymptomatic and was prescribed an oral anticoagulant (warfarin), ACE inhibitor, beta-blockers, and statins. At 1-year follow-up, he remained symptom-free.
Discussion
The patient in the present case report had a thrombosis in his right upper limb, and after efforts to identify the possible etiology, only a LASP was found. Therefore, the hypothesis of an embolic event in which the thrombus had formed at the site of the LASP was considered. An SP forms when the septum primum and septum secundum fuse. It is known as a LASP when the fusion is limited to the caudal portion, a right SP when the fusion limited to the cranial portion, and a double SP if the fusion is limited to the central portion. Adhesions can result in closure of an SP [2,3].
The free wall of an SP has 2 distinct layers of endocardium, which are separated by transverse muscle fibers and connective tissue. The atrial wall is morphologically typical of an atrium. In the apex there is an accumulation of sub-endocardial connective tissue. The presence of muscular tissue can allow contractions and emptying of blood out of the lumen [2]. TEE with contrast seems to be the criterion standard for detecting LASP (better than computed tomography) because it can unambiguously detect the PFO channel. Therefore, TEE with microbubbles is the best imaging modality for diagnosing SP [5].
To date, the literature about LASP has been focused on its association with stroke, especially cryptogenic stroke [6,7], which makes sense because the majority of the thrombi from the left heart go to the brain [1]. However, thrombi can originate in a LASP and they can go to any vessel [8]. Therefore, a patient with a LASP can have a non-stroke embolic event. Because a LASP is connected to the systemic circulation, this anatomical entity first was considered a source of thrombi and linked to cardioembolic events [8], and was later hypothesized to be a thrombogenic site for stroke [7]. However, a meta-analysis performed by Strachinaru et al [9] in 516 stroke patients and 779 controls showed no difference in LASP prevalence between the controls and the patients who had experienced an ischemic stroke (hazard ratio [HR] 1.20; 95% confidence interval [Cl] 0.96–1.53;
Today, it is understood that not all patients with an LASP have an increased risk of embolic events [10,11]. The laminar blood flow coming from the right pulmonary veins is a protective mechanism against thrombus formation along the interatrial septum. This protection can be lost in the setting of conditions such as atrial fibrillation (AF), mitral stenosis, high ventricular pressure, heart failure, and anomalies in pulmonary vein confluence, leading to risk of blood stasis and thrombus formation [3,11]. Thrombotic masses emerging from an LASP have been described in a patient who had significant risk factors for blood stasis [12]. Therefore, the association between LASP and several clinical conditions could increase the chance of embolic events.
According to a meta-analysis by Holda [7] that included 400 patients with cryptogenic strokes and 1456 controls without stroke, the risk of cryptogenic stroke was higher in those with than without a LASP (odds ratio 1.52; 95% CI 1.15–2.00;
It is important to remember that the first documented case of SP involved embolization to the left circumflex artery. There are many unmet needs regarding LASP, and an important one is determining whether it is a risk factor for non-stroke embolic events. This task is difficult, but it is possible that an SP could be the cause of an embolic events for which the etiology is otherwise not apparent.
Conclusions
The aim of our report was to raise awareness about the thrombogenic potential of LASP and the possibility of its association with an embolic event in the upper limbs of patients who have no significant risk factors for blood stasis. Because a LASP can be associated with non-stroke embolic events, physicians should consider this possibility, especially when a patient has such an event and its etiology is unknown. There are no specific clinical recommendations related to the anatomic structure of a LASP.
Figures
References:
1.. Krishnan SC, Slazar M, Septal pouch in the left atrium: JACC Cardiovasc Interv, 2010; 3; 98-104
2.. Mazur M, Jasinska KA, Walocha JA, The morphology, clinical significance and imaging methods of atrial septal pouch: A critical review: Translat Res Anatomy, 2018; 13; 7-11
3.. Hołda MK, Krawczyk-Ożóg A, Koziej M, Left-sided atrial septal pouch is a risk factor for cryptogenic stroke: J Am Soc Echocardiogr, 2018; 31(7); 771-76
4.. Kuwaki H, Takeuchi M, Kaku K, Thrombus attached to the left atrial septal pouch assessed on 3 dimensional transesophageal echocardiography: Circ J, 2011; 57(9); 2280-81
5.. Hołda MK, Krawczyk-Ożóg A, Koziej M, Cardiac computed tomography compared with two-dimensional transesophageal echocardiography for the detection and assessment of atrial septal pouches: Int J Cardiovasc Imaging, 2018; 34(8); 1305-13
6.. Wong JM, Lombardo DM, Barseghian A, Left atrial septal pouch in cryptogenic stroke: Front Neurol, 2015; 6; 57
7.. Holda MK, Koziej M, Left sided atrial septal pouch as a risk factor of cryptogenic stroke: A systematic review and meta-analysis: Cerebrovasc Dis, 2018; 46; 223-29
8.. Kirchhof P, Benussi S, Kotecha D, 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: Eur Heart J, 2016; 37; 2893-62
9.. Strachinaru M, Castro Rodriguez J, Verbeet T, Gazagnes MD, The left atrial septal pouch as a risk factor for stroke: A systematic review: Arch Cardiovasc Dis, 2017; 357; 2262-68
10.. Tugcu A, Okajima K, Jin Z, Septal pouch in the atrium and risk of ischemic stroke: A case-control study: JACC Cardiovasc Imaging, 2010; 3; 1276-83
11.. Zisa D, Faletra FF, Wessler BS, Ridges and pouches: A case series of anomalous atrial septal fusion, 2019; 4(1); 7-17
12.. Ohanyan A, Cuminetti G, Morissens M, Beware of the LASP! A structure with thrombogenic potential: Echocardiography, 2020; 37(1); 152-53
13.. Hołda MK, Koziej M, Wszołek K, Left atrial accessory appendages, diverticula, and left-sided septal pouch in multi-slice computed tomography. Association with atrial fibrillation and cerebrovascular accidents: Int J Cardiol, 2017; 244; 163-68
Figures
In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250