06 March 2022: Articles 
The Missing Link: A Case of Severe Adverse Reaction to Histamine in Food and Beverages
Unknown etiology, Challenging differential diagnosis
József Tamasi1ABDEF*, Zsuzsanna Balla2BDE, Dorottya Csuka2BD, László Kalabay1DE, Henriette Farkas2BDEDOI: 10.12659/AJCR.934212
Am J Case Rep 2022; 23:e934212
Abstract
BACKGROUND: Adverse reaction to histamine found in food and beverages is still a debated entity. It presents with a diverse, multisystemic clinical picture and lacks objective diagnostic criteria.
CASE REPORT: We report a case of severe adverse reaction to histamine in food and beverages. The 36-year-old White man had diet-dependent problems for 17 years that involved periodic erythematous rash, fever, headaches, nausea, and upper respiratory symptoms. The symptoms developed in the same chronologic order each time. The course of the disease could be divided into a first (prodromal, gastrointestinal), a second (acute, dermal), and a third (subacute, respiratory) phase. The symptoms occurred every 3-6 weeks and lasted for 10-14 days. The differential diagnosis was time-consuming and very detailed. Family history, genetic testing, and oral histamine provocation testing supported the diagnosis of an adverse reaction to histamine in food and beverages. A low-histamine diet resulted in a symptom-free state. Follow-up lasted longer than 24 months.
CONCLUSIONS: This presentation of an adverse reaction to histamine in food and beverages can serve as a textbook example where a chronological, syndrome-like order of symptom appearance is described. To the best of our knowledge, this is the first report of a severe adverse reaction to histamine in food and beverages, where symptoms are described in 3 distinct recurring phases.
Keywords: Amine Oxidase (Copper-Containing), food intolerance, Histamine, Adult, Beverages, Food Hypersensitivity, Headache, Humans, Male, Syndrome
Background
Histamine and histamine receptors have an extensive role in regulating physiological functions throughout the body. An adverse reaction to histamine (ARH) in a susceptible person is caused by the ingestion of non-toxic amounts of histamine.
Two enzymes are responsible for the inactivation of exogenous histamine: diamine oxidase (DAO) and histamine N-methyltransferase (HNMT). A decreased function of these enzymes may have a wide-ranging effect. The skin, the gastrointestinal system (eg, diarrhea), the immune system, the respiratory system (eg, allergy and asthma-like symptoms), the nervous system (eg, headaches) [1], the psyche, and the cardiovascular system [2] may be affected.
Histamine is produced by microorganisms from the amino acid histidine. It is most likely not the lone provoking agent for an ARH. Other biogenic amines may further complicate the pathology. Also, some foods naturally contain high levels of histamine and other biogenic amines.
ARH in food and beverages is a debated entity. It lacks standardized diagnostic means and is a diagnosis of exclusion at present. The often multifaceted, non-reproducible, subjective presentations make clinical evaluation a challenge [3]. ARH is frequently referred to as “histamine intolerance”. However, a consensus on the use of this phrase has not been reached.
Differential diagnosis consists of many possibilities. Allergies, pseudo-allergies, other intolerances, mastocytosis, mast cell leukemia, alpha tryptasemia, mast cell activation syndrome, secondary histamine intolerance (eg, caused by medication), and hereditary angioedema have to be taken into consideration.
ARH has a well-documented resemblance to scombroid poisoning [4]. Scombroid poisoning is caused by toxic amounts of histamine and (likely) other biogenic amines. In ARH, symptoms are caused by insufficient capacity to degrade histamine. These 2 are linked together throughout our case presentation.
Case Report
A 36-year-old man presented with episodes of recurrent dry flushing that occurred every 3-6 weeks. The redness was diffuse, non-palpable, non-pruritic, and blanchable. It involved the neck, the chest, the inner arms, and the inner thighs, but it never included the face. Urticaria or wheals were not present.
Additional patient concerns followed the same pattern each time, by which the course of the disease could be divided into 3 phases. The first (prodromal or gastrointestinal) phase lasted for 2 days. Symptoms included nausea, malaise, fatigue, fever (up to 38.7°C axillary), lack of appetite, and occasionally emesis. A pulsating, unilateral headache sometimes developed and persisted for up to 7 days.
The second (acute or dermal) phase was rash-predominant and lasted for 24–36 hours (Figures 1–3). The erythematous rash was accompanied by loose stools, abdominal cramps, sore throat, and promptly-improving prodromal symptoms.
The third and longest (subacute or respiratory) phase was characterized by a sore throat, rhinorrhea, mild coughing, and headaches. The upper respiratory symptoms persisted for up to 2 weeks, often leading to purulent rhinitis.
In the 2–4 weeks of the inter-attack intervals, symptoms occasionally occurred solely or in conjunction for short stretches. They, at times, foretold the development of the next rash-dominated period.
Symptoms started within 5 hours after meals, and never on an empty stomach. They developed mostly in the afternoon or at night. Depressed mood accompanied the onset of the symptoms and the patient was absent from work for 1–5 days.
Previously unmentioned symptoms included heartburn, muscle twitching, postprandial sleepiness, sneezing, and phantosmia (smelling smoke) after meals. A typical meal that provoked an attack was fish soup with wine (others included aged cheese, pizza, seafood, fried liver, pickled vegetables, tomato, and eggplant). Antihistamines and H2 receptor antagonists lessened the symptoms to some degree.
The patient had 3 siblings with similar symptoms and 1 without any health issues. Two brothers (aged 34 and 31) presented a very similar clinical picture (Figures 4–6). These brothers presented all 3 phases of an ARH. Leading symptoms were gastrointestinal in one case and asthma-like in the other case. The youngest sister (aged 23) presented the first and the third phase, with headaches as the leading symptom. Disease onset was as a young adult in all the affected siblings. It started as a recurring sore throat in all cases. None of the siblings had any comorbidities.
The patient had no other medical conditions or allergies. As part of the diagnostic effort, several specialists (Table 1) examined him and detailed laboratory testing was done, without any pathological findings (suppl. table with raw laboratory data). Differential diagnosis ruled out hereditary angioedema (HAE) with laboratory (Table 2) and genetic testing (SERPING1, plasminogen, factor XII).
Low serum DAO activity (relevant results in Table 3) was present. Single-nucleotide polymorphism (SNP) tests for DAO and HNMT were also performed (Table 4). The histamine 50-Prick test was positive, with a wheal size of 3.5 mm after 20 and 50 minutes.
A food and symptom diary led to the suspected diagnosis of an ARH found in food and beverages. A low-histamine diet was introduced, which resulted in an entirely symptom-free state. Single, mild symptoms (heartburn, diarrhea, headache, phantosmia) could be provoked by obvious dietary mistakes and confirmed through repeated provocation with the suspected food.
Oral histamine provocation was conducted after several months on a low-histamine diet. The test was done very cautiously with 8, 15, and 25 mg of histamine dihydrochloride dissolved in 200 ml of water. Eight mg did not provoke symptoms. Fifteen mg provoked irritation of the oral mucosa, nausea, headaches, rhinorrhea, and depressed mood. In the next 4 days, muscle twitching, slight headaches after meals, loose stools, bloating, and heartburn occurred. Twenty-five mg caused sneezing and a sore throat in conjunction with the previously mentioned symptoms.
After 17 months of being symptom-free, several tests were redone to exclude any undiagnosed comorbidities (Table 1). To avoid unnecessary dietary restrictions, diet was adjusted to the individual tolerance levels. Follow-up lasted longer than 24 months.
Discussion
ARH is described as a multisystemic disease with seemingly unrelated symptoms. This case represents the missing link in understanding the chronological order of symptom appearance. The patient displays symptoms connected to the gastrointestinal tract in the (first) prodromal phase due to the ingestion of exogenous histamine. The symptoms of the acute (second) phase were likely due to the generalized effects of histamine, just as in scombroid poisoning. The subacute (third) phase involved the respiratory system.
A severe susceptibility to ingested histamine may present with symptoms comparably serious to scombroid poisoning, but the duration is different. The course of the disease in scombroid poisoning is time-limited due to a functioning histamine inactivation. Both may display nausea, vomiting, diarrhea, cramping, respiratory symptoms, headaches, and erythema [5].
The latest “guideline on management of suspected adverse reactions to ingested histamine” states that an ARH lacks objective testing methods [6]. Testing for levels of histamine in plasma is not a reliable method. Histamine has a very short plasma half-life, and fluctuates during the day [7]. Also, intra-cellular histamine elevation cannot be measured.
The validity of serum DAO activity (which should be preferred over serum DAO concentration [8]) is not supported by enough evidence. Therefore, diagnosis based on this result is not possible. Measuring the levels of DAO cofactors total copper and vitamin B6 can be helpful.
SNP tests for DAO and HNMT aided identification of a primary origin. Our patient was a heterozygotic carrier of DAO SNPs rs2052129 (GT), rs2268999 (AT), and rs10156191 (CT). These can result in reduced serum DAO activity [9].
The patient was a carrier of rs1050891 SNP (AA genotype), which results in lower HNMT activity [10]. The patient was not a carrier of HNMT polymorphism rs11558538 (C314T).
This results in a Thr105Ile amino acid substitution that leads to decreased levels of HNMT enzymatic activity [11].
The histamine pathway operates with more than 200 genes coding for enzymes and other proteins. More SNPs may also be involved in an ARH, but presently only these tests have evidence supporting their validity.
Results of an oral histamine provocation test and histamine 50-Prick test supported the diagnosis [12]. The latter is usually done with 0.5–1 mg/kg of histamine dihydrochloride (40–80 mg). Nevertheless, low doses were enough to establish the diagnosis.
Complexity is a serious obstacle to understanding cause-and-effect relationships between symptoms and diet [13]. Histamine does not alter smell, appearance, or taste, it is heat-stable, and remains present after freezing or canning. Freshness, preparation, and storage all affect an ARH. Certain food additives, beverages, drugs, and other biogenic amines can also cause problems [14]. Careful surveillance was indispensable [15]. It was observed that certain foods lead to symptoms depending on quantities. Some foods (eg, meat) were not harmful when prepared fresh but caused problems after storage.
The symptomless state after the introduction of a low-histamine diet was conclusive in the diagnostic process [16]. Allergies and other intolerances were satisfactorily excluded with a thorough diagnostic effort [17].
Ruling out HAE was crucial in the differential diagnosis. Patients with HAE often have a positive family history. HAE triggers include alcohol, infection, mental stress, surgical procedures, and medication [18]. Quality of life is strongly impacted [19]. Prodromal symptoms include fatigue, gastrointestinal flu-like symptoms, and erythema marginatum. Gastrointestinal attacks may present with abdominal pain, nausea, vomiting, and diarrhea. These may be a result of bowel wall edema. HAE can be divided into subgroups depending on the levels of C1 inhibitor (C1-INH): HAE with normal levels of C1-INH and HAE with C1-INH deficiency (type 1 and 2). The latter (C1-INH-HAE) is caused by the autosomal dominant mutation of the SERPING1 gene encoding C1-inhibitor [20]. Patients with HAE may show abnormal complement system laboratory findings [21], but routine laboratory tests show no abnormalities.
Limitations of the diagnostic effort include not performing intestinal DAO and HNMT activity testing [22]. Access to these tests is very limited. Furthermore, there is no explanation of why the usual laboratory and genetic tests of this condition did not reflect the severity of the case. Provocation with such low doses of oral histamine did not lead to the wide-ranging scale of symptoms.
Conclusions
In a severe susceptibility, an adverse reaction to ingested histamine seems to take a cyclical path with distinct, recurring periods. This case is a good textbook example with all possible symptoms on display. More research is needed to understand why most patients only show limited symptoms. As the pathology is unknown, the underlying cause for cyclicality remains unexplained. This case also highlights the lack of objective diagnostic methods.
Figures
Tables
Table 1.. Examinations performed in the differential diagnostic prodcedure.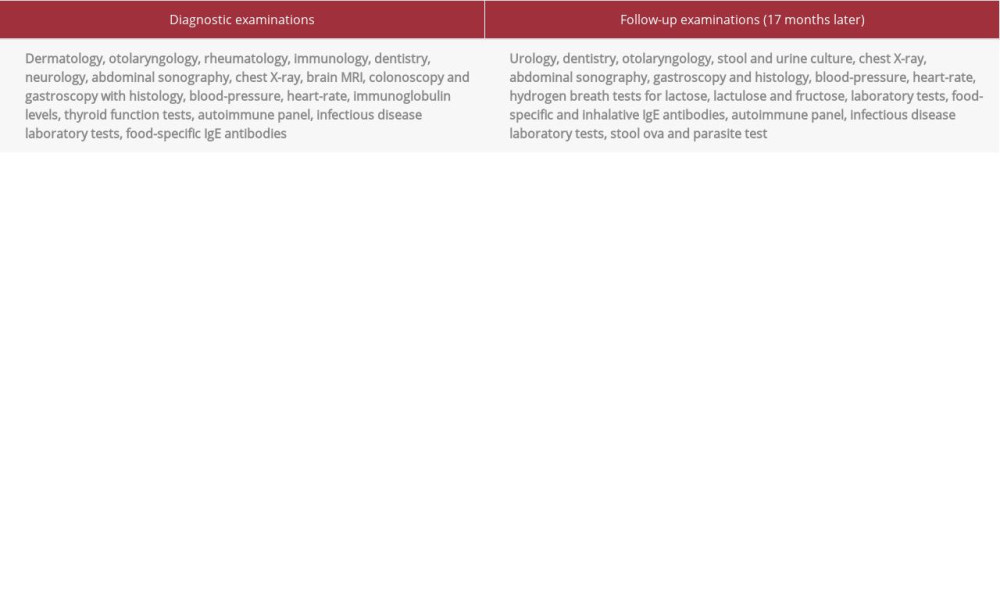 Table 2.. Laboratory tests for hereditary angioedema.
Table 2.. Laboratory tests for hereditary angioedema.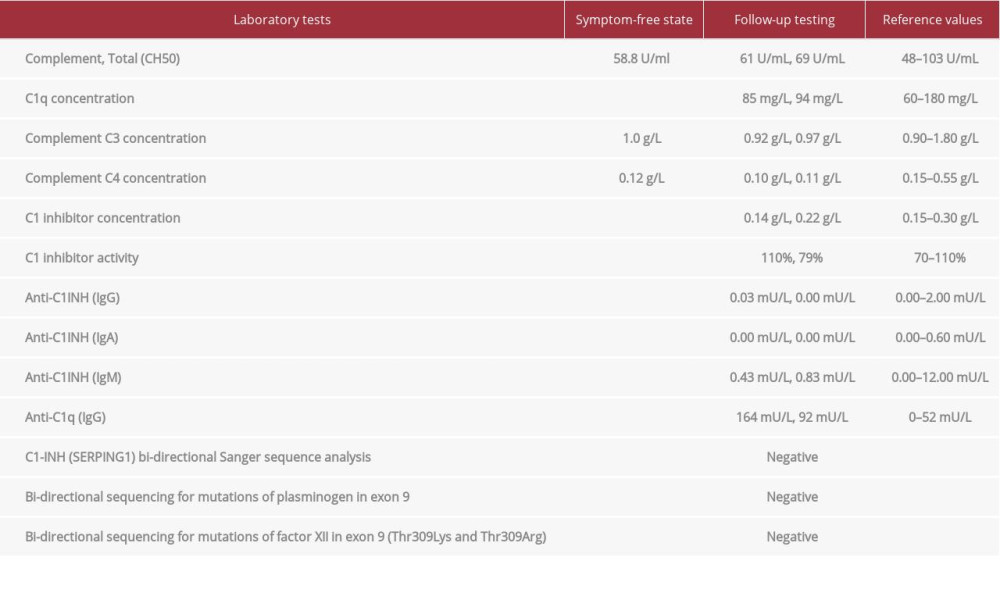 Table 3.. Relevant laboratory tests in case of an adverse reaction to histamine found in food and beverages.
Table 3.. Relevant laboratory tests in case of an adverse reaction to histamine found in food and beverages.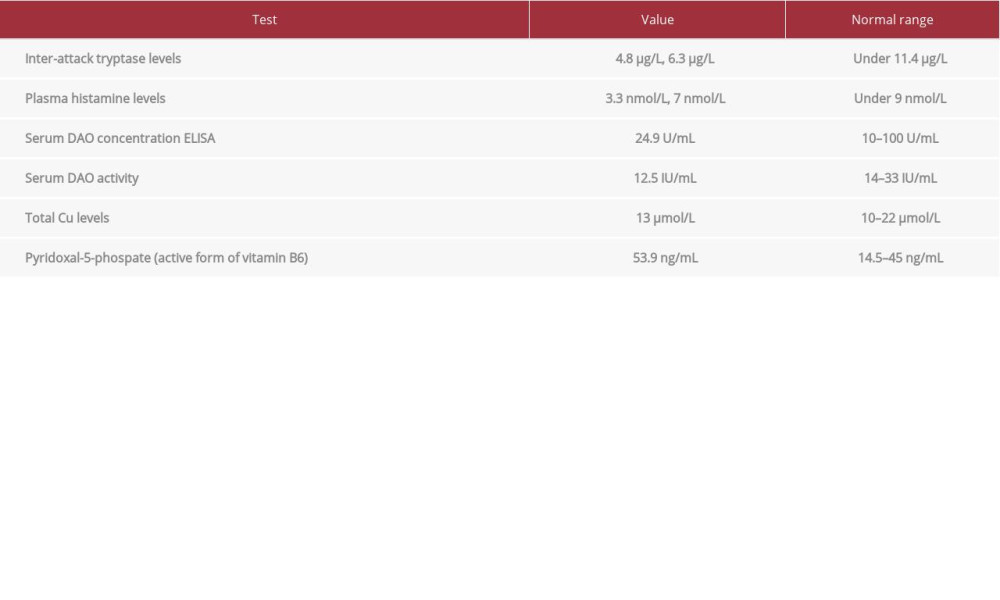 Table 4.. Single-nucleotide polymorphisms of DAO and HNMT.
Table 4.. Single-nucleotide polymorphisms of DAO and HNMT.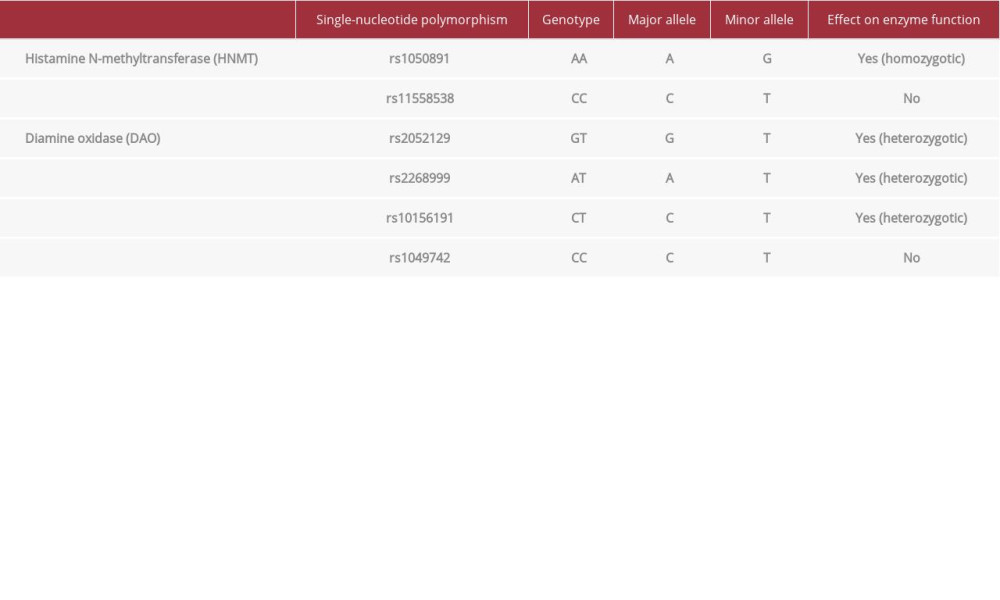
References:
1.. Izquierdo-Casas J, Comas-Basté O, Latorre-Moratalla ML, Low serum diamine oxidase (DAO) activity levels in patients with migraine: J Physiol Biochem, 2018; 74(1); 93-99
2.. Solymosi D, Sárdy M, Pónyai Gy, [Biogenic amine (histamine) intolerance, associated symptoms, comorbidities in the dermatology-allergy practice]: Borgyogy Venerol Sz, 2020; 96(1); 11-20 [in Hungarian]
3.. Schnedl WJ, Lackner S, Enko D, Evaluation of symptoms and symptom combinations in histamine intolerance: Intest Res, 2019; 17(3); 427-33
4.. Hungerford JM, Histamine and Scombrotoxins: Toxicon, 2021; 201; 115-26
5.. Rodríguez-Caravaca G, Hijas-Gómez AI, Tejedor-Alonso MÁ, Food poisoning caused by scombroids: A case-control study: J Infect Public Health, 2019; 12(4); 591-93
6.. Reese I, Ballmer-Weber B, Beyer K, Guideline on management of suspected adverse reactions to ingested histamine: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergology and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA) as well as the Swiss Society for Allergology and Immunology (SGAI) and the Austrian Society for Allergology and Immunology (ÖGAI): Allergol Select, 2021; 5(1); 305-14
7.. Pinzer TC, Tietz E, Waldmann E, Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls: Allergy, 2018; 73(4); 949-57
8.. Manzotti G, Breda D, Di Gioacchino M, Serum diamine oxidase activity in patients with histamine intolerance: Int J Immunopathol Pharmacol, 2016; 29(1); 105-11
9.. Maintz L, Yu CF, Rodríguez E, Association of single nucleotide polymorphisms in the diamine oxidase gene with diamine oxidase serum activities: Allergy, 2011; 66(7); 893-902
10.. Kim SH, Kang YM, Kim SH, Histamine N-methyltransferase 939A>G polymorphism affects mRNA stability in patients with acetylsalicylic acid-intolerant chronic urticaria: Allergy, 2009; 64(2); 213-21
11.. Pang YP, Zheng XE, Weinshilboum RM, Theoretical 3D model of histamine N-methyltransferase: insights into the effects of a genetic polymorphism on enzymatic activity and thermal stability: Biochem Biophys Res Commun, 2001; 287(1); 204-8
12.. Wagner A, Buczyłko K, Zielińska-Bliźniewska H, Impaired resolution of wheals in the skin prick test and low diamine oxidase blood level in allergic patients: Postepy Dermatol Alergol, 2019; 36(5); 538-43
13.. Shulpekova YO, Nechaev VM, Popova IR, Food intolerance: The role of histamine: Nutrients, 2021; 13(9); 3207
14.. Schnedl WJ, Enko D, Histamine intolerance originates in the gut: Nutrients, 2021; 13(4); 1262
15.. Solymosi D, Sárdy M, Pónyai G, Interdisciplinary significance of food-related adverse reactions in adulthood: Nutrients, 2020; 12(12); 3725
16.. Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, Histamine intolerance: The current state of the art: Biomolecules, 2020; 10(8); 1181
17.. Schnedl WJ, Enko D, Considering histamine in functional gastrointestinal disorders: Crit Rev Food Sci Nutr, 2021; 61(17); 2960-67
18.. Zotter Z, Csuka D, Szabó E, The influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiency: Orphanet J Rare Dis, 2014; 9; 44
19.. Balla Z, Ignácz B, Varga L, How angioedema quality of life questionnaire can help physicians in treating C1-inhibitor deficiency patients?: Clin Rev Allergy Immunol, 2021; 61(1); 50-59
20.. Farkas H, Dóczy A, Szabó E, Screening for plasminogen mutations in hereditary angioedema patients: Genes, 2021; 12(3); 402
21.. Farkas H, Veszeli N, Kajdácsi E, “Nuts and bolts” of laboratory evaluation of angioedema: Clin Rev Allergy Immunol, 2016; 51(2); 140-51
22.. Reese I, Nutrition therapy for adverse reactions to histamine in food and beverages: Allergol Select, 2018; 2(1); 56-61
Figures
Tables
 Table 1.. Examinations performed in the differential diagnostic prodcedure.
Table 1.. Examinations performed in the differential diagnostic prodcedure. Table 2.. Laboratory tests for hereditary angioedema.
Table 2.. Laboratory tests for hereditary angioedema. Table 3.. Relevant laboratory tests in case of an adverse reaction to histamine found in food and beverages.
Table 3.. Relevant laboratory tests in case of an adverse reaction to histamine found in food and beverages. Table 4.. Single-nucleotide polymorphisms of DAO and HNMT.
Table 4.. Single-nucleotide polymorphisms of DAO and HNMT. Table 1.. Examinations performed in the differential diagnostic prodcedure.
Table 1.. Examinations performed in the differential diagnostic prodcedure. Table 2.. Laboratory tests for hereditary angioedema.
Table 2.. Laboratory tests for hereditary angioedema. Table 3.. Relevant laboratory tests in case of an adverse reaction to histamine found in food and beverages.
Table 3.. Relevant laboratory tests in case of an adverse reaction to histamine found in food and beverages. Table 4.. Single-nucleotide polymorphisms of DAO and HNMT.
Table 4.. Single-nucleotide polymorphisms of DAO and HNMT. In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








