28 January 2022: Articles 
Secondary Hemophagocytic Lymphohistiocytosis in a Neonate with SARS-CoV-2 Infection
Challenging differential diagnosis, Rare disease, Clinical situation which can not be reproduced for ethical reasons
Hasan Zuhair El-IsaDOI: 10.12659/AJCR.934839
Am J Case Rep 2022; 23:e934839
Abstract
BACKGROUND: Infants born to mothers with Coronavirus disease (COVID-19) are susceptible to infection, either vertically or horizontally. The mechanism is not completely understood. Regardless, it is rare that an infant with COVID-19 suffers from serious, life-threatening complications. We speculate that one of these complications can be hemophagocytic lymphohistiocytosis (HLH), a dysregulated hyperinflammatory response that leads to multi-organ failure.
CASE REPORT: We describe a case of a female newborn, born to a SARS-CoV-2-positive mother via cesarean section delivery at 35 weeks of gestation, that tested positive for SARS-CoV-2 on the first day after birth. The patient presented with progressive respiratory distress, intermittent fever, splenomegaly, and cytopenia. Hemophagocytic lymphohistiocytosis (HLH) work-up was done, which showed hyperferritinemia, hypofibrinogenemia, and hypertriglyceridemia. Bone marrow aspirate showed hemophagocytic activity of both red blood cells and platelets. We suspect that the virus triggered HLH, which led to the patient’s death at 51 days of age due to severe respiratory failure.
CONCLUSIONS: Infants and children suffer from milder symptoms than adults when infected with SARS-CoV-2, for reasons not well understood, although multiple hypotheses have been proposed, which are discussed in this paper. However, there is a possibility that the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) virus can cause HLH and multi-system inflammatory syndrome in children (MIS-C).
Keywords: COVID-19, Infant, Newborn, Lymphohistiocytosis, Hemophagocytic, Pediatric Multisystem Inflammatory Disease, COVID-19 Related, Respiratory Distress Syndrome In Premature Infants, COVID-19, Cesarean Section, Female, Humans, Pregnancy, Pregnancy Complications, Infectious, SARS-CoV-2, Systemic Inflammatory Response Syndrome
Background
Hemophagocytic lymphohistiocytosis (HLH) is a rare but fatal condition caused by a dysregulated hyperinflammatory response and immune activation with elevated levels of inflammatory cytokines that can lead to multi-organ involvement and marked hemophagocytosis resulting in ferritin elevation and glycosylated ferritin decline due to macrophage activation, and cytopenia [1,2]. Although HLH can occur at any age, it predominantly affects infants younger than 18 months [3]. HLH is usually categorized into 2 subtypes: primary (familial) HLH and secondary (acquired) HLH. Although both primary and secondary HLH have similar presentation and both can be triggered by viral infections, particularly Epstein-Barr virus, they differ in the presence or absence of genetic predisposition. Primary/familial HLH has a genetic predisposition that impairs natural killer (NK) or T cell function, while secondary HLH (sHLH) is mostly associated with concomitant infections, whether viral or bacterial, autoimmune diseases, or underlying malignancies [1]. Similarly, Macrophage activation syndrome (MAS) is a condition that shares some features of HLH, such as strong macrophage activation and excessive hyperferritinemia. MAS is associated with autoimmune diseases, especially juvenile idiopathic arthritis, systemic lupus erythematosus (SLE), Kawasaki disease, and juvenile dermatomyositis [4].
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the Coronavirus disease 2019 (COVID-19) pandemic, emerged in Wuhan, China, in December 2019. The first case of SARS-CoV-2 infection appeared in March 2020 and reached a peak number of daily cases in November 2020, which led the virus to become widespread throughout Jordan. Children with COVID- usually experience milder symptoms than adults, but they are susceptible to the same complications. However, current evidence suggests that infants (age <1 year) might be at increased risk for severe illness from SARS-CoV-2 infection [5]. Although there is no previous evidence of SARS-CoV-2 being the main etiology of HLH per se in children, there is good evidence that SARS-CoV-2 is involved in the development of the systemic hyperinflammatory state known as multi-system inflammatory syndrome (MIS-C), which can mimic the symptoms of Kawasaki disease in children [6]. In this paper we present a case of an infant with a consistent feature of sHLH secondary to severe SARS-CoV-2 infection.
Case Report
We report a female patient who was born via cesarean section delivery at 35 weeks of gestation due to intrauterine growth retardation and fetal distress to a SARS-CoV-2-positive mother diagnosed by nasopharyngeal swab real-time polymerase chain reaction (PCR) testing. At birth, the baby was small for gestational age, with a birth weight of 1.690 Kg. The one-minute and five-minute Apgar scores were 8 and 9, respectively. The infant was kept in a designated isolated neonatal negative pressure room with enhanced infection control precautions where staff were required to wear N95 masks, eye shields, gloves, and gowns. She was started on antibiotics (ampicillin and amikacin) for 2 days after obtaining blood for bacterial cultures to treat suspected sepsis. A work-up was done for TORCH (
The patient developed late-onset sepsis at age 6 days. Blood and urine bacterial and fungal cultures were collected; blood culture revealed
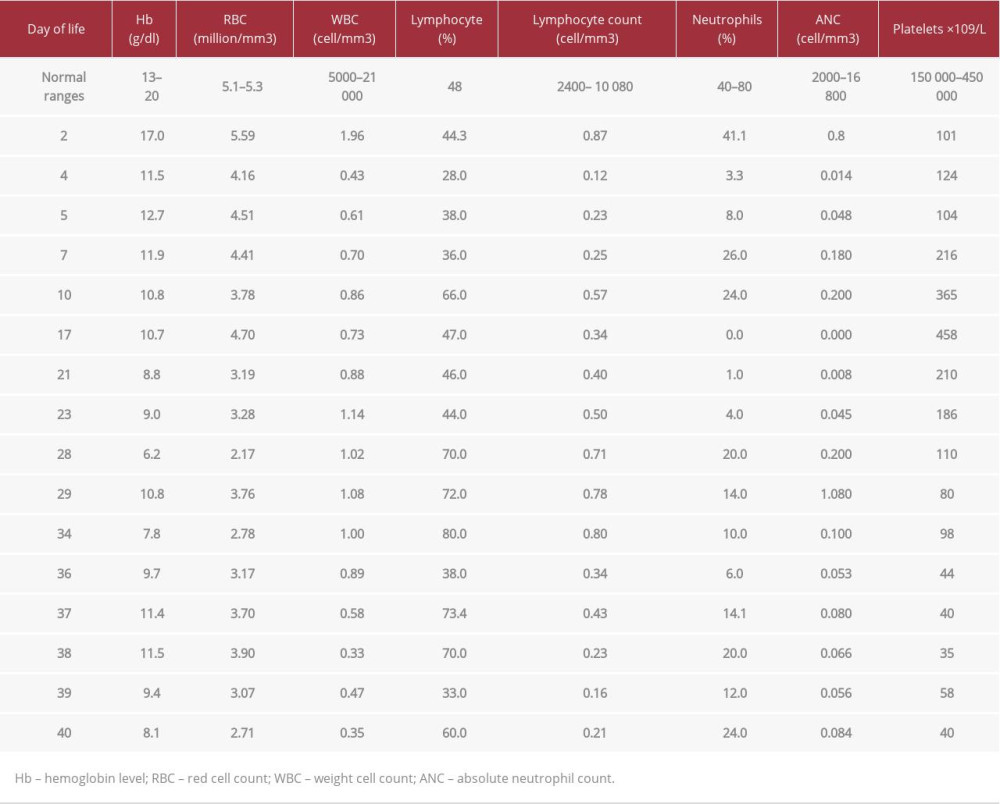
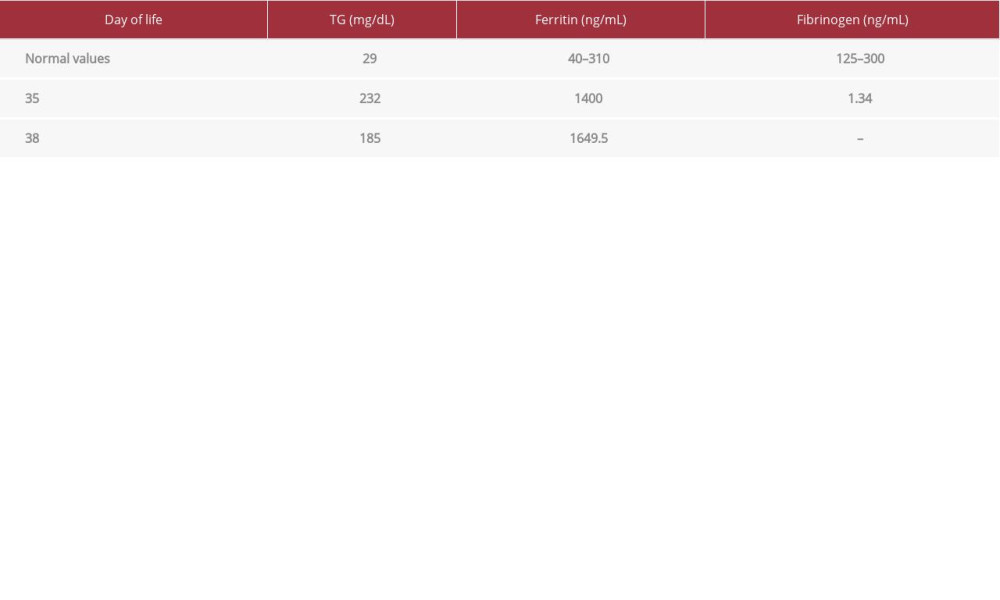
At age 40 days, the patient started to have an intermittent fever and worsening of respiratory status, with an increase in her FiO2 requirement. This was followed by splenomegaly at age 46 days (documented by ultrasound). Her chest X-ray showed diffuse bilateral infiltrate (Figure 2). An echocardiogram showed mild to moderate pulmonary hypertension secondary to lung disease. One week later, the patient was managed by invasive respiratory support, antibiotics, granulocyte colony-stimulating factor (G-CSF), and a 2-day course of intravenous immunoglobulin (IVIg). An HLH work-up was done, which showed hyperferritinemia, hypofibrinogenemia, and hypertriglyceridemia (Table 2). A bone marrow aspirate sample was taken since a biopsy was not possible, and it showed hemophagocytic activity of both red blood cells and platelets (Figure 3). Peripheral blood film did not show blasts, which excluded leukemias. The patient died at age 51 days due to severe respiratory failure.
Discussion
We present the case of an infant with confirmed SARS-CoV-2 infection born to a mother with known perinatal SARS-CoV-2 infection (SARS-CoV-2-positive sample at 24 h after birth, when the mother was also positive). Recent reports suggest that, for unknown reasons, children have a milder disease course than adults, and children often do not require hospitalization or any medical intervention [6]. The reason for this observation is not well understood, yet multiple hypotheses have been proposed. One of these hypotheses is that children have immature angiotensin-converting enzyme 2 (ACE2) protein, and evidence suggests that ACE2 protein is needed by SARS-CoV-2 to enter the cells; as a result, well-differentiated cells that express mature ACE2 protein are readily infected by the virus [7] a large number of SARS-related coronaviruses (SARSr-CoVs. Another hypothesis suggests that the presence of fetal hemoglobin, which makes up 80% of an infant’s hemoglobin, has a prophylactic effect against SARS-CoV-2, as the virus usually targets the heme on the 1-β chain (which is not present in fetal hemoglobin) of hemoglobin, dissociating the iron from porphyrin. This leads to the destruction of hemoglobin, which leads to hypoxia as well as inhibiting the normal metabolic pathway of heme [8].
It has been described that severe COVID-19 can lead to systemic hyperinflammation indicative of secondary HLH in adults. The mechanism by which sHLH occurs may involve SARS-CoV-2 activating NLRP3 inflammasomes, the interaction between the spike glycoprotein of SARS-CoV-2 and Toll-like receptors 5, and downregulation of the antiproliferative and anti-inflammatory AT-1–7 pathway [9–11]. HLH is diagnosed either by detection of HLH-associated mutations (diagnostic for primary HLH) or by verification of a minimum of 5 of the following 8 criteria: prolonged high-grade fever, hepatosplenomegaly, accompanying cytopenia, fasting hypertriglyceridemia (> 265 mg/dl), and/ or hypofibrinogenemia (< 150 mg/dl), hemophagocytosis, low or absent NK cell activity, ferritin > 500 ng/ml, and soluble IL-2 receptor elevated 2 standard deviations above age-adjusted laboratory-specific norms [12].
Some patients (especially those with underlying diseases, infants less than 1 year of age, and neonates) are more prone to serious outcomes [13]. Neonates are likely to differ from older groups in their exposure to the virus; infection with neonatal admission following birth to a mother with perinatal SARS-CoV-2 infection was reported to be unlikely, and vertical transmission is rare, although they can contract SARS-CoV-2 through close personal contact in much the same way as other groups. They might also contract the virus vertically before or at birth [14]. For diagnosis in newborns, PCR is reliable in the detection of SARS-CoV-2 in neonates, but the possibility of false-negative results is high. This may be because the incubation period during the first 2 days of life is too short [15].
We present one of the first reported neonatal deaths with severe COVID-19 course in Jordan. Although our case most likely demonstrates vertical transmission, as various reports suggest this possibility in neonates [16–18], due to the PCR test being positive within 24 h from birth, horizontal transmission cannot be ruled out. The patient was born at 35 weeks of gestation due to intrauterine growth restriction (IUGR). According to a clinical analysis of 10 neonates born to mothers with SARS-CoV-2 infection, 2 of the newborns were small for gestational age, suggesting that SARS-CoV-2 infection may be associated with SGA, and that infections in general may be associated with IUGR [19]. A review of the literature found that various pre-natal congenital infections, such as those caused by
Several reports demonstrate the occurrence of HLH after severe COVID-19 disease in adults. Hypertriglyceridemia (associated with lipoprotein lipase inhibition caused by excess tumor necrosis factor-alpha [TNF-α]) is found in ~36–71% of adults with HLH [23–25].
Although there is currently no definitive evidence of in-utero SARS-CoV-2 transmission, in addition to unavailable serological evidence of SARS-CoV-2 with elevated immunoglobulin-M levels (which were not documented at the time of negative PCR testing), the present case report draws attention to the possibility of the complication of HLH in infants with confirmed infection born to mothers with known perinatal SARS-CoV-2 infection who had a severe course of COVID-19. Patients should be screened for hyperinflammation using standard laboratory tests to identify those for whom immunosuppressive therapy may improve the outcomes. Regarding the delivery method when the mother is SARS-CoV-2-positive, according to a systematic review, cesarean section in mothers positive for SARSCoV-2 has the same incidence of SARS-CoV-2 infection, neonatal death, and maternal death as in vaginal delivery [26].
In a small subset of patients, a hyperinflammatory syndrome known as multi-system inflammatory syndrome in children (MIS-C) can occur. Case series have reported that children who present with this hyperinflammatory syndrome have fever and mucocutaneous manifestations that resemble those seen in Kawasaki disease. Other reports have described patients with features suggestive of macrophage activation syndrome, toxic shock syndrome, and hemophagocytic lymphohistiocytosis. MIS-C predominantly affects children older than 5 years of age and it is relatively rare in those less than 1 year of age [27]. The diagnostic criteria were described as any individual under age 21 years presenting with fever (>38.0°C for ≥24 h), laboratory evidence of inflammation (such as an elevated C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], elevated neutrophils, or reduced lymphocytes), evidence of clinically severe illness requiring hospitalization and involving 2 or more organ systems in the setting of a SARS-COV-2 infection (current or recent by RT-PCR, serology, or antigen test), with no plausible alternative diagnosis [28].
Patients with MIS-C have been reported to present with secondary hemophagocytic lymphohistiocytosis (sHLH), a rare but fatal hyperinflammatory syndrome that usually affects those who are less than 18 months old. Our patient was found to have 5 of the 8 diagnostic criteria of HLH, which is sufficient for the diagnosis, but no genetic testing was done to rule out primary HLH in this patient. However, as the diagnostic criteria of MIS-C were fulfilled, in the background of a positive SARS-COV-2 test, we found it more plausible that this was a case of secondary HLH, with the main culprit being the active SARS-CoV-2 infection. Our patient was managed by invasive respiratory support, antibiotics, granulocyte colony-stimulating factor (G-CSF), and a 2-day course of intravenous immunoglobulin (IVIg). The diagnosis of HLH was established at age 46 days, IVIg was given first, and when steroids were considered, it was too late, as the patient died shortly thereafter. Before the diagnosis of HLH, steroids were avoided due to the positive blood cultures and fear of bacteremia complications.
Thus, this case shows that, although rare, SARS-COV-2 can have disastrous consequences in children, especially in premature neonates who have intrauterine growth retardation. Therefore, in the current COVID-19 pandemic, obstetricians and neonatologists should take the utmost precautions when delivering a fetus of a mother who is positive for COV-SARS-2 to prevent both horizontal and vertical transmission of the virus. With a presentation such as our patient’s, MIS-C would be suspected, which would make the diagnosis of HLH challenging; therefore, it is important to note that we were only able to diagnose the patient after the exclusion of MIS-C and performing a bone marrow aspirate.
Conclusions
In conclusion, this case report highlights that although it is extremely rare, MIS-C can occur in the neonates with a clinical picture of secondary hemophagocytic lymphohistiocytosis (sHLH).
Figures
References:
1.. Mehta P, McAuley DF, Brown M, COVID-19: Consider cytokine storm syndromes and immunosuppression: Lancet, 2020; 395(10229); 1033-34
2.. Lambotte O, Cacoub P, Costedoat N, High ferritin and low glycosylated ferritin may also be a marker of excessive macrophage activation: J Rheumatol, 2003; 30(5); 1027-28
3.. Esteban YM, de Jong JLO, Tesher MS, An overview of hemophagocytic lymphohistiocytosis: Pediatr Ann, 2017; 46(8); e309-13
4.. Lin C-I, Yu H-H, Lee J-H, Clinical analysis of macrophage activation syndrome in pediatric patients with autoimmune diseases: Clin Rheumatol, 2012; 31(8); 1223-30
5.. Dong Y, Mo X, Hu Y, Epidemiology of COVID-19 among children in China: Pediatrics, 2020; 145(6); e20200702
6.. Patel NA, Pediatric COVID-19: Systematic review of the literature: Am J Otolaryngol, 2020; 41(5); 102573
7.. Zhou P, Yang X-L, Wang X-G, A pneumonia outbreak associated with a new Coronavirus of probable bat origin: Nature, 2020; 579(7798); 270-73
8.. Rawat M, Chandrasekharan P, Hicar MD, Lakshminrusimha S, COVID-19 in newborns and infants-low risk of severe disease: Silver lining or dark cloud?: Am J Perinatol, 2020; 37(8); 845-49
9.. Zhang C, Wu Z, Li J-W, Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality: Int J Antimicrob Agents, 2020; 55(5); 105954
10.. Tholin B, Hauge MT, Aukrust P, Hemophagocytic lymphohistiocytosis in a patient with COVID-19 treated with tocilizumab: A case report: J Med Case Rep, 2020; 14(1); 187
11.. Hakim NN, Chi J, Olazagasti C, Liu JM, Secondary hemophagocytic lymphohistiocytosis versus cytokine release syndrome in severe COVID-19 patients: Exp Biol Med, 2020; 246(1); 5-9
12.. Henter J-I, Horne A, Aricó M, HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis: Pediatr Blood Cancer, 2007; 48(2); 124-31
13.. Götzinger F, Santiago-García B, Noguera-Julián A, COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study: Lancet Child Adolesc Heal, 2020; 4(9); 653-61
14.. Bhattacharjee S, Banerjee M, Pal R, COVID-19 associated hemophagocytic lymphohistiocytosis and coagulopathy: Targeting the duumvirate: Indian Pediatr, 2020; 57(9); 827-33
15.. Bahadur G, Bhat M, Acharya S, Retrospective observational RT-PCR analyses on 688 babies born to 843 SARS-CoV-2 positive mothers, placental analyses and diagnostic analyses limitations suggest vertical transmission is possible: Facts Views Vis Obgyn, 2021; 13(1); 53-66
16.. Zeng L, Xia S, Yuan W, Neonatal early-onset infection with SARSCoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China: JAMA Pediatr, 2020; 174(7); 722-25
17.. Alzamora MC, Paredes T, Caceres D, Severe COVID-19 during pregnancy and possible vertical transmission: Am J Perinatol, 2020; 37(8); 861-65
18.. Hu X, Gao J, Luo X, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumonia: Obstet Gynecol, 2020; 136(1); 65-67
19.. Zhu H, Wang L, Fang C, Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia: Transl Pediatr, 2020; 9(1); 51-60
20.. Longo S, Borghesi A, Tzialla C, Stronati M, IUGR and infections: Early Hum Dev, 2014; 90(Suppl. 1); S42-44
21.. Gale C, Quigley MA, Placzek A, Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: A prospective national cohort study using active surveillance: Lancet Child Adolesc Heal, 2021; 5(2); 113-21
22.. Felsenstein S, Hedrich CM, SARS-CoV-2 infections in children and young people: Clin Immunol, 2020; 220; 108588
23.. Otrock ZK, Eby CS, Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis: Am J Hematol, 2015; 90(3); 220-24
24.. Barba T, Maucort-Boulch D, Iwaz J, Hemophagocytic lymphohistiocytosis in Intensive Care Unit: A 71-case strobe-compliant retrospective study: Medicine (Baltimore), 2015; 94(51); e2318
25.. Apodaca E, Rodríguez-Rodríguez S, Tuna-Aguilar EJ, Demichelis-Gómez R, Prognostic factors and outcomes in adults with secondary hemophagocytic lymphohistiocytosis: A single-center experience: Clin Lymphoma Myeloma Leuk, 2018; 18(10); e373-80
26.. Cai J, Tang M, Gao Y, Cesarean section or vaginal delivery to prevent possible vertical transmission from a pregnant mother confirmed with COVID-19 to a neonate: A systematic review: Front Med (Lausanne), 2021; 8; 634949
27.. Chiotos K, Bassiri H, Behrens EM, Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: A case series: J Pediatric Infect Dis Soc, 2020; 9(3); 393-98
28.. , Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with coronavirus disease 2019 (COVID-19). HAN Archive – 00432
Figures
In Press
04 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.941835
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943042
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250