11 July 2022: Articles 
A Case Series of Lymphatic Injuries After Suction Lipectomy in Women with Lipedema
Unusual clinical course, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Clinical situation which can not be reproduced for ethical reasons
Thomas F. Wright1ABCDEF*, Karen L. Herbst2CDEFDOI: 10.12659/AJCR.935016
Am J Case Rep 2022; 23:e935016
Abstract
BACKGROUND: Lipedema is a loose connective tissue disease characterized by disproportionate subcutaneous adipose tissue hypertrophy in the extremities. There is evidence of impaired lymphatic function in women with lipedema at all stages without signs of trophic skin changes associated with hereditary or acquired lymphedema. A modification of suction lipectomy is used to treat lipedema tissue and can reduce pain, limb size, and limb swelling and reduce the need for compression in women with lipedema. Studies have shown that modified liposuction can improve quality of life and mobility. There are no reports of lymphatic injury after suction lipectomy in patients with lipedema in PubMed indexed journals.
CASE REPORT: Three women with lipedema who had no prior venous or lymphatic disease developed new-onset symptomatic International Society of Lymphology (ISL) Stage 2 or 3 lymphedema and skin and tissue changes within 6 months to 1 year after suction lipectomy for lipedema tissue on the legs. Each of the 3 women had their surgeries performed using different suction devices and under different types of anesthesia. Two of the lymphatic injury cases had subsequent nuclear lymphoscintigrams that confirmed impaired lymphatic function.
CONCLUSIONS: We report 3 cases of women with lymphatic injuries after modified suction lipectomy to treat lipedema. Clinical history, exams, and confirmatory studies support the assessment that suction lipectomy caused newly-manifested signs and symptoms of lymphedema. Further study is needed to determine the risk of permanent lymphatic injury with suction lipectomy in larger numbers of lipedema patients.
Keywords: Lipectomy, Lipedema, Lymphatic Abnormalities, Edema, Female, Humans, lymphedema, Quality of Life
Backgrond
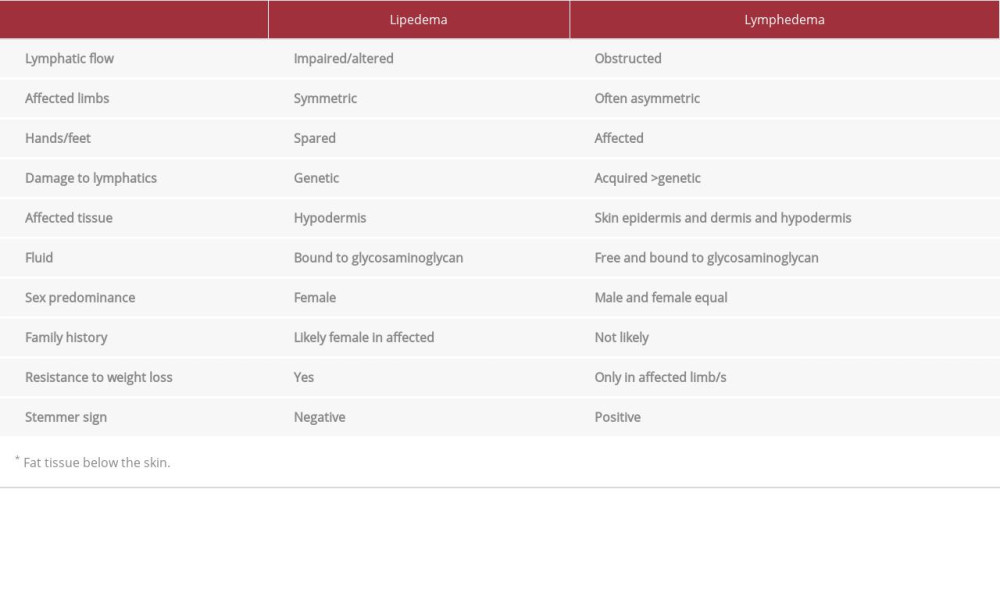
Lipedema is a symmetric, chronic, progressive loose connective tissue (LCT) disease occurring primarily in women. Altered LCT in lipedema results in swelling of the limbs and deposition of glycosaminoglycans (GAGs) and fibrosis in the inter-stitial matrix [1,2]. Lipedema is disproportionate, meaning the swelling and accumulation of abnormal LCT is much greater on the legs and/or arms than the rest of the body. Lipedema progression is graded by stage. Stage 1 lipedema is characterized by a thickening and disproportionate accumulation of LCT in the extremities. The skin remains smooth, but there are small palpable nodules in the LCT; the tissue is generally not heavy or swollen, but there is often pain and tissue resistance to loss by diet or exercise. Stage 2 lipedema is characterized by increased fibrous tissue leading to larger nodules in the LCT and increased swelling and tenderness of affected areas. Stage 3 is characterized by increased body mass index, the formation of lobules of skin and LCT, and larger and more extensive masses in the tissue [3]. The exact timing of and cause of progression of lipedema through stages is not known but it typically advances over decades. A secondary lymphedema, lipolymphedema, can occur especially in later stages of lipedema [4,5].
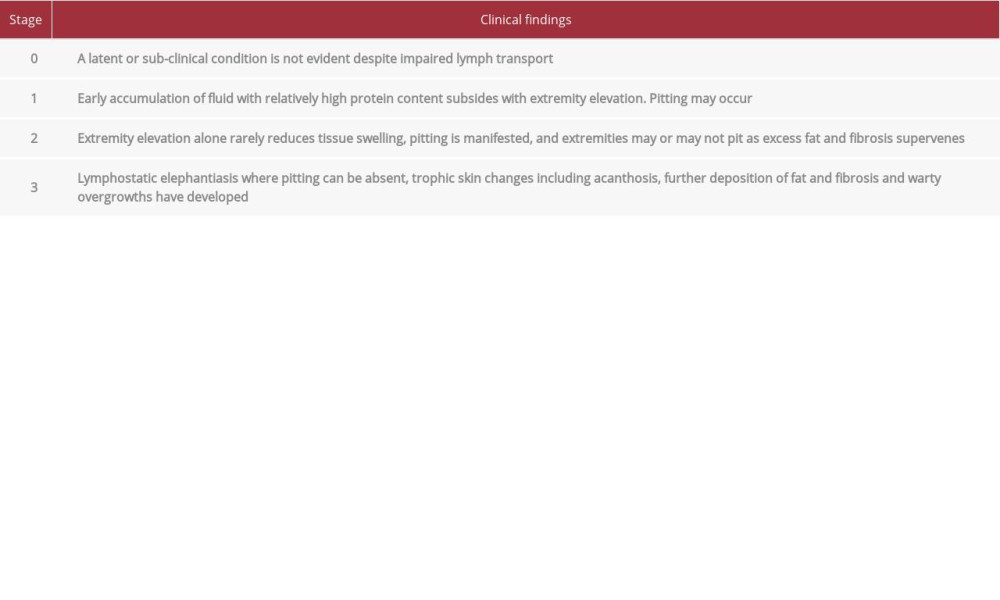
Impairment of lymphatic transport by Klienhan’s transport index is found in 47% and 63% of patients with lipedema, [4,6] but lipedema remains distinct from lymphedema in that the lymphatic system is not obstructed. Bioimpedance spectroscopy studies found increased extracellular water, magnetic resonance imaging (MRI) of lipedema LCT demonstrated increased sodium content and extracellular fluid, but ultrasound did not show free fluid as in lymphedema, consistent with increased fluid and sodium bound to GAGs in the extracellular matrix [5,7–9]. All patients with lymphedema have abnormal lymphatic function, which closely corelates with their disease progression and severity. As lymphedema progresses, it affects all 3 layers of the skin – the epidermis, dermis, and hypodermis – while lipedema pathology is seen mostly in the hypodermis. Stage 3 lymphedema is distinguished by trophic skin changes of dermal fibrosis principally papillomatosis and hyperkeratosis, as indicated in Table 1 [10].
In lipedema, fibrosis is generally limited to the hypodermis and usually does not cause a dermal trophic skin changes unless it is complicated by secondary lymphedema, as indicated in Table 2.
Suction lipectomy (liposuction) is one of the most common cosmetic procedures performed worldwide, including in the United States (U.S.) [11]. Generally, cosmetic suction lipectomy is performed on healthy individuals with normal lymphatic function who are close to ideal weight. A derivative or modification of suction lipectomy procedures that use cannulas attached to suction to remove adipose and other subcutaneous tissue components are also increasingly used as a medically necessary non-cosmetic surgical treatment of lymphedema [12] and lipedema [9]. The application of suction cannulas for these diseases removes not only subcutaneous adipose tissue but also other LCT components, including extracellular proteins and GAGs. The proper term for removal of all the components of loose connective tissue to improve lymphatic function is fibro-lympho-lipo-aspiration (FLLA) [11], a modified suction lipectomy, which has been shown to improve lymphatic function and symptoms of lymphatic impairment and reduce the need for decongestive treatment in lymphedema and lipedema [9,13–15]. Studies show no change or improvement in lymphatic function associated with lipedema after modified suction lipectomy [6,9].
In the U.S., there are currently several surgical suction devices used to treat lipedema, including a water-assisted suction liposuction (WAL) device that incorporates a pressurized jet of tumescent fluid with the suction cannula, power-assisted liposuction (PAL) that has an electrically powered handpiece that vibrates the suction cannula, and ultrasound-assisted liposuction (UAL) that delivers ultrasound energy to liquify the tissue prior to suction.
Hoffmann et al reported that lymphatic collecting vessels can be injured with and without tumescent solution during modified suction lipectomy [16]. Reporting of intermediate and long-term complications of surgical procedures in the medical literature is suboptimal. In January 2022, PubMed was searched for the following terms to determine if there were any reports on lymphatic injury after modified suction lipectomy: Suction Lipectomy, Liposuction, Lymphatic Injury, Lymphedema, Lipedema, Lipoedema. There are no reports, prior to the cases reported here, of lymphatic injury from modified suction lipectomy in patients with lipedema. We describe 3 cases of women with lymphatic injuries after modified suction lipectomy to treat lipedema, who presented within a 3-month period to a community-based venous and lymphatic specialist (author) for additional treatment.
Case Reoprts
CASE 1:
A 55-year-old woman noticed her legs were larger than normal going through puberty. She noticed that her legs were tender, bruised easily, and that her symptoms notably worsened with each pregnancy. At 47 years of age, when she started going through menopause, she gained additional weight and her leg tenderness worsened. Weight loss programs resulted in weight loss in the trunk and little or no improvement to the legs. She reported a 33 Kg weight loss after exercise programs but disproportionate loss with minimal change to the lower half of her body. She had no history or symptoms consistent with lymphedema or venous insufficiency. Her exam showed an overall healthy woman, with BMI 38 kg/m2, with disproportionately large arms and legs. Her exam revealed soft skin with multiple evenly dispersed palpable subcutaneous nodules on her arms and legs. The excess subcutaneous tissue stopped at the wrist and ankle, creating the appearance of cuffs; there were no skin lobules or signs of lymph-edema. She underwent UAL under general anesthesia. A total of 6 liters of suction aspirate was removed from her arms and legs. Her immediate postoperative period was uncomplicated. Postoperative complications that developed over the following year included dermal fibrosis, dermal papillomatosis with warty growths on the skin, mottled skin, and persistent swelling in her medial and anterior thighs (Figures 1, 2). She was diagnosed with Stage 2 lipedema on her arms and legs using the Wold criteria [17] and Stage 3 secondary lymphedema according to the ISL Staging System [10] by a lipedema and lymphatic specialist. Skin changes consistent with lymphedema included dermal fibrosis, hyperkeratosis, and dermal papillomatosis with multiple verrucous nodules on the anterior medial thighs and upper calves.
Lymphoscintigram showed bilateral delayed lymphatic flow with poor visualization of lymphatic vessels and uptake in both inguinal lymph nodes by 1 h after injection. At 3 h after injection, lymphatic vessels were seen with full uptake of Technetium-99, consistent with bilateral delayed lymphatic function (Figure 3).
CASE 2:
A 38-year-old woman developed symmetrical disproportionate subcutaneous fat accumulation on her hips and thighs during puberty. Her legs and hips increased in size through her 20s. She reported her legs were heavy and tender and bruised easily. Approximately 6 months prior to presentation, she had suction lipectomy with PAL under general anesthesia on her hips and thighs. Over the 6-month postoperative period, she developed progressive right foot and ankle swelling. She had been wearing postoperative compression daily that included compression shorts and a thigh-high medical grade compression stocking on her right leg. She had no personal or family history of venous or lymphatic disease. Her overall appearance was that of a healthy young woman with BMI 30.4 kg/ m2. She had lobules on her lateral thighs and her inner thighs had swollen disproportionate subcutaneous tissue compared to her trunk. There were palpable walnut-size nodules on her hips and thighs. Her right foot had trace pitting edema and there was 1+ non-pitting edema from her ankle to her thigh on her right leg. She had dermal fibrosis and dermal sclerosis on the top of her right foot with mild hyperkeratosis at the flexure of her ankle. Measurements of her legs revealed 1.5 cm increase in circumference at the right ankle. She had a positive Kaposi-Stemmer sign on her right foot and negative Kaposi-Stemmer sign on her left foot (Figure 4). She was diagnosed with Type 2, Stage 2 Lipedema of the lower body and ISL Stage 2 lymphedema in her right leg [10] by a lipedema and lymphatic specialist.
Lymphoscintigram showed early visualization of lymphatic vessels and uptake on the left leg and delayed lymphatic flow and delayed and diminished uptake in the inguinal lymph nodes on the right leg, consistent with right leg lymphatic obstruction (Figure 5).
CASE 3:
A 62-year-old woman presented with symmetric, disproportionately enlarged subcutaneous tissue on her legs since puberty. She reported her legs are tender, bruise easily, with symptoms notably worsening during menopause. Weight loss programs resulted in only loss of tissue from the trunk and little or no improvement of the legs. A 23-kg weight loss after bariatric surgery at age 50 resulted in minimal change to the lower half of her body. She had no history of lymphatic disease. Prior to suction lipectomy, an exam revealed a woman with BMI 28.3 kg/m2 and legs notable for soft subcutaneous nodules and no sign of lymphedema. She was diagnosed with Stage 2 lipedema and underwent 3 suction lipectomy surgeries. She had WAL of her arms and calves to ankles, with a total of 6 liters of aspirate removed, of which 5350 cc was primarily fat. Six months later, she had WAL on her inner and anterior thighs, hips, and knees with a total removal of 7200 cc of aspirate, with 6700 cc being primarily fat. After an additional 6 months, she had WAL suction lipectomy to her knees, lower posterior thighs, and ankles, and 1800 cc of total aspirate was removed; the supernatant was 1400 cc. Over the year following her last surgery, she developed persistent dermal sclerosis, dermal hyperkeratosis, persistent pigment irregularities, and intermittent swelling in her lateral left ankle and lower leg (Figure 6). Her diagnosis of lipedema was confirmed by a lipedema and lymphatic specialist following criteria by Wold [17]. She was also diagnosed with Stage 2 lymphedema by ISL criteria [10].
All patients provided consent to sharing their data and photographs consistent with the Declaration of Helsinki. Cases were enrolled consecutively.
Discussion
PRESENTATION:
As referral centers for lipedema and lymphedema, we regularly see women with lipedema who have developed complications after suction lipectomy. Most of the skin complications from suction lipectomy previously reported start with skin hyperemia and or pallor followed by skin necrosis and ulceration during the early postoperative period and or are associated with infection and or poor skin healing. The complications reported herein were not associated with any of these complications in the early postoperative period. The skin changes reported here only became apparent after 6 months to one year following surgery, meeting the definition of chronic skin changes and swelling (lymphedema).
The cases of women with lipedema presented here did not have any history, signs, or symptoms of lymphedema prior to modified suction lipectomy. After the surgery, all 3 patients developed symptoms and trophic skin changes consistent with a diagnosis of lymphedema by ISL Staging criteria [10]. Dermal skin changes of fibrosis, sclerosis, hyperkeratosis, and papillomatosis are the defining clinical signs for Stage 2 or 3 lymph-edema (Table 1). While dermal sclerosis and fibrosis can occur with several injury mechanisms, dermal hyperkeratosis and papillomatosis are only seen with advanced lymphedema and dermal papillomatosis is caused by fibrosis around an injured lymphatic vessels [20].
LYMPHEDEMA AND CONFIRMATORY LYMPHOSCINTIGRAPHY:
All 3 patients showed clear clinical signs and met the ISL criteria for lymphedema, confirmed by lymphoscintigraphy in 2 of the 3 cases (the 3rd was lost to follow-up and did not have a lymphoscintigram performed to confirm the clinical diagnosis). The first case had bilateral delayed lymphatic flow and radionucleotide uptake while the second case had classic right-sided lymphatic impairment of lymphatic flow with uni-lateral delayed uptake at the inguinal lymph nodes.
EXPECTED BENEFITS OF LIPEDEMA REDUCTION SURGERY:
In patients with normal lymphatic function, provided there is not a severe injury to the lymphatic collectors, the lymphatic system almost always recovers and returns to a normal state. Suction lipectomy with small blunt cannulas and surgical techniques that focus on avoiding lymphatic damage have been reported to halt lipedema progression [14,21,22]. A modification of suction lipectomy can result in alleviating or at least improving the swelling, leg heaviness and fatigue, the need for limb compression, and the need for lymphatic massage in women with lipedema [14,15,23,24]. Van de Pas showed that careful suction lipectomy improved radionucleotide inguinal uptake on lymphoscintigraphy [13]. In patients with an impaired lymphatic function such as chronic lymphedema (or lipedema), careful suction lipectomy using techniques to avoid lymphatic injury can result in improved lymphatic function and a decreased rate of secondary infection or cellulitis in the affected limbs [12,24]. These reported benefits did not occur in these 3 cases.
The question becomes whether the development of lymphedema was the result of variations of surgical technique or technology, or the higher risk of lymphatic injury in lipedema or a combination of these factors.
POSSIBLE INJURY MECHANISMS:
Blunt suction lipectomy using a dry technique can cause injury to lymphatic collectors. In a study analyzing the lower extremity of fresh postmortem cadavers, blunt cannula suction lipectomy caused either no injury or moderate injury to lymphatic collectors using a longitudinal technique [16]. Utilizing a transverse technique caused moderate to severe lymphatic injury. With tumescent anesthesia applied prior to suction-assisted lipectomy, the longitudinal technique showed either no injury or moderate injury, and the transverse technique did not show severe injury; the use of tumescence therefore protects the tissue against damage but was not sufficient protection for the cases reported herein.
Reports exist of dilated, aneurysmal, and tortuous lymphatic collectors in the limbs of women with lipedema, especially in later stages, and there is evidence of microvascular inflammation and altered lymphatics in lipedema [2,21,22,25]. These variations would likely pose an increased risk of lymphatic injury to patients with lipedema.
ULTRASONIC-ASSISTED TECHNOLOGY:
One of the 3 case reports herein that had lymphatic and dermal complications of suction lipectomy had UAL. The ultrasonic-assisted technology adds energy that is converted to heat and can increase the risk of lymphatic injury. The standard of care guidelines of the United Kingdom, Germany, the Netherlands, and Spain for the treatment of lipedema all recommend against the use of additional energy during suction lipectomy [25–28].
ANESTHESIA:
Two of the 3 cases also utilized general as well as tumescent anesthesia. It is possible that the use of general anesthesia can prevent feedback from the patient about an area of inadequate tumescent anesthesia, which could increase the risk of lymphatic injury. The Dutch guidelines on suction lipectomy treatment of lipedema recommend against using general anesthesia for the treatment of lipedema. Previous studies have shown that liposuction under general anesthesia and/ or with relatively little subcutaneous infiltration is contraindicated for lipedema because of the substantial risk of causing damage to the lymphatic system [20,29].
Conclusions
Three women with lipedema developed lymphedema after suction lipectomy procedures. Improved reporting of complications and longitudinal studies from surgeons using modifications of suction lipectomy are needed to know the relative risk of postsurgical lymphatic complications and other complications in women with lipedema.
Figures
References:
1.. Felmerer G, Stylianaki A, Hägerling R, Adipose tissue hypertrophy, an aberrant biochemical profile and distinct gene expression in lipedema: J Surg Res, 2020; 253; 294-303
2.. AL-Ghadban S, Cromer W, Allen M, Dilated blood and lymphatic microvessels, angiogenesis, increased macrophages, and adipocyte hypertrophy in lipedema thigh skin and fat tissue: J Obes, 2019; 2019; 8747461
3.. Torre YS, Wadeea R, Rosas V, Herbst KL, Lipedema: Friend and foe: Horm Mol Biol Clin Investig, 2018; 33(1); 2017-0076
4.. Forner-Cordero I, Olivan-Sasot P, Ruiz-Llorca C, Munoz-Langa J, Lymphoscintigraphic findings in patients with lipedema: Rev Esp Med Nucl Imagen Mol, 2018; 37(6); 341-48
5.. Cellina M, Gibelli D, Soresina M, Non-contrast MR Lymphography of lipedema of the lower extremities: Magn Reson Imaging, 2020; 71; 115-24
6.. Gould DJ, El-Sabawi B, Goel P, Badash I, Uncovering lymphatic transport abnormalities in patients with primary lipedema: J Reconstr Microsurg, 2019; 23(10); 0039-1697904
7.. Crescenzi R, Marton A, Donahue PMC, Tissue sodium content is elevated in the skin and subcutaneous adipose tissue in women with lipedema: Obesity (Silver Spring), 2018; 26(2); 310-17
8.. Iker E, Mayfield CK, Gould DJ, Patel KM, Characterizing lower extremity lymphedema and lipedema with cutaneous ultrasonography and an objective computer-assisted measurement of dermal echogenicity: Lymphat Res Biol, 2019; 7(10); 525-30
9.. Peprah K, MacDougall D: Liposuction for the treatment of lipedema: A review of clinical effectiveness and guidelines, 2019, Ottawa (ON), Canadian Agency for Drugs and Technologies in Health
10.. , The Diagnosis and Treatment of Peripheral Lymphedema: 2016 Consensus Document of the International Society of Lymphology: Lymphology, 2016; 49(4); 170-84
11.. Chia CT, Neinstein RM, Theodorou SJ, Evidence-based medicine: Liposuction: Plast Reconstr Surg, 2017; 139(1); 267e-74e
12.. Campisi CC, Ryan M, Boccardo F, Campisi C, Fibro-lipo-lymph-aspiration with a lymph vessel sparing procedure to treat advanced lymphedema after multiple lymphatic-venous anastomoses: The complete treatment protocol: Ann Plast Surg, 2017; 78(2); 184-90
13.. van de Pas CB, Boonen RS, Stevens S, Does tumescent liposuction damage the lymph vessels in lipoedema patients?: Phlebology, 2020; 35(4); 231-36
14.. Schmeller W, Hueppe M, Meier-Vollrath I, Tumescent liposuction in lipoedema yields good long-term results: Br J Dermatol, 2012; 166(1); 161-68
15.. Rapprich S, Baum S, Kaak I, Kottmann T, Podda M, Treatment of lipoedema using liposuction. Results of our own surveys: Phlebologie, 2015; 44; 121-32
16.. Hoffmann JN, Fertmann JP, Baumeister RG, Tumescent and dry liposuction of lower extremities: Differences in lymph vessel injury: Plast Reconstr Surg, 2004; 113(2); 718-24 ; discussion 725–26
17.. Wold LE, Hines EA, Allen EV, Lipedema of the legs; A syndrome characterized by fat legs and edema: Ann Intern Med, 1951; 34(5); 1243-50
18.. Dixit VV, Wagh MS, Unfavourable outcomes of liposuction and their management: Indian J Plast Surg, 2013; 46(2); 377-92
19.. Wright T, Herbst KL: Results of REDCap survey of self-identified lipedema surgeons in the US, 2020
20.. DiSipio T, Rye S, Newman B, Hayes S, Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis: Lancet Oncol, 2013; 14(6); 500-15
21.. Sandhofer M, Hanke CW, Habbema L, Prevention of progression of lipedema with liposuction using tumescent local anesthesia: Results of an International Consensus Conference: Dermatol Surg, 2020; 46(2); 220-28
22.. Rapprich S, Dingler A, Podda M, Liposuction is an effective treatment for lipedema-results of a study with 25 patients: J Dtsch Dermatol Ges, 2011; 9(1); 33-40
23.. Beninson J, Edelglass JW, Lipedema – the non-lymphatic masquerader: Angiology, 1984; 35(8); 506-10
24.. Tiedjen KU, Holzmann H, Altmeyer P, Hör G: Dermatologie und Nuklearmedizin, 1985; 432-38, Berlin, Springer-Verlag [in German]
25.. Reich-Schupke S, Schmeller W, Brauer WJ, S1 guidelines: Lipedema: J Dtsch Dermatol Ges, 2017; 15(7); 758-67
26.. Halk AB, Damstra RJ, First Dutch guidelines on lipedema using the international classification of functioning, disability and health: Phlebology, 2017; 32(3); 152-59
27.. Alcolea JM, Alonso Alvarez B, Arroyo Bielsa A, Documento de Consenso Lipedema 2018: 33rd National Congress of the Spanish Society of Aesthetic Medicine (SEME), 2018, Malaga, Spain. Barcelona, Spain, LITOGAMA S.L.
28.. Coppel T, Cunneen J, Fetzer S, Best practice guidelines: The management of lipoedema: Wounds UK [Internet], 2017 Available from:http://www.wounds-uk.com/best-practice-statements/best-practice-guidelines-the-management-of-lipoedema
29.. Forner-Cordero I, Szolnoky G, Forner-Cordero A, Kemény L, Lipedema: An overview of its clinical manifestations, diagnosis and treatment of the dis-proportional fatty deposition syndrome – systematic review: Clin Obes, 2012; 2(3–4); 86-95
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250