15 February 2022: Articles 
Folinic Acid, Fluorouracil, and Oxaliplatin Therapy for Recurrent Esophageal Cancer with Syndrome of Inadequate Antidiuretic Hormone Secretion (SIADH) After Preoperative Cisplatin/5-Fluorouracil Therapy
Unusual or unexpected effect of treatment, Rare disease
Ryoko Futai1A*, Tomoo Yoshie1B, Tsuyoshi Sanuki1B, Yuta Inoue1B, Tetsuyuki Abe1B, Ayaka Sasaki1B, Takao Iemoto1B, Hiroki Hayashi1B, Takayuki Ose1B, Teruhisa Morikawa1BDOI: 10.12659/AJCR.935121
Am J Case Rep 2022; 23:e935121
Abstract
BACKGROUND: Cisplatin/5-fluorouracil therapy is the standard therapy for unresectable and recurrent esophageal cancer. Cisplatin-based chemotherapy often causes adverse effects, such as nausea, vomiting, and renal dysfunction, which may necessitate dose modification or treatment prolongation. Therefore, novel combination therapies are urgently needed to improve the efficacy and overcome drug toxicity in this setting.
CASE REPORT: A 77-year-old man with advanced esophageal cancer received cisplatin/5-fluorouracil therapy as neoadjuvant chemotherapy. On day 8 of administration, the patient had lightheadedness, diaphoresis, and nausea and became unconscious and developed severe hyponatremia. We diagnosed the patient with cisplatin-induced syndrome of inadequate antidiuretic hormone secretion (SIADH). Subsequently, water restriction was started, and treatment with a salt-added diet and 3% hypertonic saline infusion was initiated. The hyponatremia improved and the patient was discharged on day 16 of administration. Therefore, neoadjuvant chemotherapy was discontinued, and surgical treatment was performed. However, the tumor recurred and chemotherapy was required. The patient developed severe hyponatremia while receiving neoadjuvant chemotherapy; hence, folinic acid, fluorouracil, and oxaliplatin therapy (FOLFOX) were administered as an alternative treatment. The patient completed the FOLFOX therapy without developing SIADH.
CONCLUSIONS: The cisplatin/5-fluorouracil therapy is currently the standard chemotherapy regimen for esophageal cancer. However, SIADH is a known adverse effect when using cisplatin. In patients with esophageal cancer, oxaliplatin appears to have a lower risk of SIADH than cisplatin, suggesting that oxaliplatin can be a therapeutic option for patients with esophageal cancer who are at high risk of SIADH.
Keywords: Cisplatin, esophageal squamous cell carcinoma, Hyponatremia, oxaliplatin, Antineoplastic Combined Chemotherapy Protocols, Esophageal Neoplasms, Fluorouracil, Humans, Inappropriate ADH Syndrome, leucovorin, Male, Neoadjuvant Therapy, Neoplasm Recurrence, Local, Vasopressins
Background
Cisplatin/5-fluorouracil therapy is the standard therapy for unresectable and recurrent esophageal cancer [1]. Cisplatin-based chemotherapy often causes adverse effects, such as nausea, vomiting, and renal dysfunction, which may necessitate dose modification or treatment prolongation [2]. In general, esophageal cancer patients have a lower performance status and poorer nutrition than patients with other cancer types. Therefore, novel combination therapies are urgently needed to improve the efficacy and overcome drug toxicity in this setting. Some patients may benefit from switching to folinic acid, fluorouracil, and oxaliplatin (FOLFOX) therapy, especially those with impaired renal function or challenges with high-volume infusions [3]. A rare adverse effect of cisplatin is syndrome of inadequate antidiuretic hormone secretion (SIADH) [4]. Here, we present a case of SIADH in a patient with esophageal squamous cell cancer who was receiving cisplatin/5-fluorouracil therapy and later successfully continued treatment with FOLFOX therapy.
Case Report
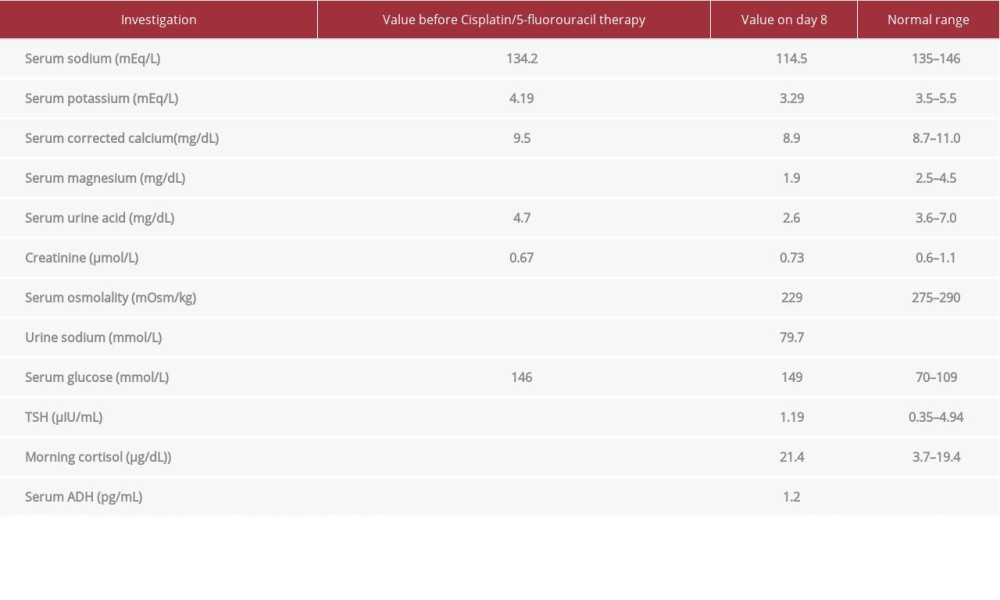
A 77-year-old man presented to the clinic with pharyngeal discomfort over the previous 2 months. An upper gastrointestinal endoscopy revealed an esophageal tumor in his thoracic esophagus. The patient was diagnosed with squamous cell carcinoma after a biopsy and was referred to our hospital. His medical history included myocardial infarction, stroke, and diabetes. His medications included aspirin, azilsartan, amlodipine besilate, indapamide, carvedilol, rosuvastatin calcium, glimepiride, sitagliptin phosphate hydrate, and voglibose. The patient did not use diuretics. Imaging studies showed no distant metastases, and a final diagnosis of cT3N0M0 stage II cancer was made. The standard care for esophageal cancer [5] is to operate after neoadjuvant chemotherapy (NAC). Cisplatin/5-fluorouracil therapy (cisplatin 80 mg/m2, 5-fluorouracil 800 mg/m2) was commenced as NAC. Hyperhydration (3000 mL/day) with mannitol and magnesium sulfate support were provided before he received cisplatin on day 1. The patient was given 200 ml of 20% mannitol injection on day 1 only. On day 8 of administration, the patient had lightheadedness, diaphoresis, and nausea and became unconscious; blood test results showed a decrease in serum sodium levels from 134 mEq/L to 106 mEq/L (135–146 mEq/L). Urine osmolality was 612 mOsm/L (50–1300 mOsm/kg), serum osmolality was 229 mOsm/L (275–290 mEq/L), fractional excretion of sodium (FENa) was 1.1%, urine Na concentration was 79.7 mEq/L, serum arginine vasopressin (AVP) was 1.2 pg/mL, and serum uric acid level was 2.6 mg/dL (3.6–7.0 mg/dL). The results of other investigations are shown in Table 1. We diagnosed the patient with cisplatin-induced SIADH as there were no abnormalities on head and chest computed tomography (CT). There was also no evidence of adrenal, thyroid, cardiac, renal, or liver dysfunction or signs of dehydration. Subsequently, water restriction was started, and treatment with a salt-added diet and 3% hypertonic saline infusion was initiated. The patient was discharged with gradual improvement in serum sodium levels to 130 mEq/L on day 16 of administration. Thoracic and abdominal contrast-enhanced CT and upper gastrointestinal endoscopy showed a marked reduction in the size of the esophageal lesion after NAC. Positron emission tomography-CT (PETCT) also showed a decrease in fluorine-18-deoxyglucose (FDG) accumulation (from 22.33 to 18.69 times the standard uptake value [SUV]), which we considered to be a minor clinical response (Figure 1A–1D). It was determined that continuing NAC was not possible because of marked hyponatremia; therefore, 1 month later, the patient underwent thoracoscopic esophagectomy, laparoscopic gastrointestinal reconstruction, and 2-field lymph node dissection with a pathological diagnosis of stage III T3N2M0 well-to-moderately differentiated squamous cell carcinoma. Four months after the surgery, a thoracoabdominal contrast-enhanced CT showed a soft tissue shadow extending from the cervical anastomosis to the superior mediastinum. PET-CT showed an accumulation of FDG at the esophageal anastomosis (19.67 times the SUV) and the cervical lymph nodes (Figure 2A, 2B), as well as at the mediastinal lymph nodes, right pleura, and 10th rib. The patient was diagnosed with recurrent esophageal cancer, right pleural dissemination, mediastinal lymph node metastasis, and rib metastasis. We decided to start the FOLFOX therapy (folinic acid 200 mg/m2, fluorouracil 2800 mg/m2, and oxaliplatin 85 mg/m2) as the patient developed marked hyponatremia with cisplatin/5-fluorouracil therapy preoperatively. The serum sodium concentration was maintained above 130 mEq/L after 1 course, and the patient was able to continue treatment without developing SIADH. Serum sodium concentration and creatinine levels before and after chemotherapy are shown in Figure 3. The patient did not develop SIADH, but contrast-enhanced CT after 4 courses of chemotherapy showed an enlarged lesion, which indicated disease progression. Chemotherapy was discontinued, and the patient received best supportive care.
Discussion
SIADH associated with advanced cancer is a common electrolyte abnormality. The frequency of SIADH (Na <130 mEq/L) has been reported to be 3.7% in patients receiving chemotherapy [6,7]. SIADH is thought to be mainly induced by platinum-based drugs, such as cisplatin, or by electrolyte abnormalities caused by vomiting, diarrhea, and massive infusions commonly associated with chemotherapy [6,7]. There are 2 possible pathophysiological mechanisms for cisplatin-induced hyponatremia – SIADH and renal salt wasting syndrome (RSWS) due to dehydration and renal dysfunction. The pathogenesis of RSWS is thought to be a tubular disorder caused by cisplatin, but the exact mechanism of action is not clear and there are no established diagnostic criteria. It is characterized by obvious signs of dehydration and renal dysfunction, resulting in marked sodium diuresis [8]. SIADH is thought to be caused by impaired electrolyte absorption at Henle’s loop, increased hydration to prevent renal damage during cisplatin administration, and accelerated antidiuretic hormone secretion (ADH) secretion due to vomiting and pain.
The concept of SIADH was proposed by Schwartz et al in 1957. It is a syndrome in which abnormal secretion of ADH causes water retention, resulting in hyponatremia [9]. There have been reports of SIADH caused by vincristine, cyclophosphamide, and cisplatin as well as other antineoplastic agents [4]. The SIADH diagnostic criteria include: 1) preserved adrenal, thyroid, renal, and cardiac function, with no cirrhosis, diuretics, and dehydration; 2) urine osmolality >100 mOsm/L; 3) serum osmolality <280 mOsm/L; 4) serum sodium concentration <135 mEq/L; 5) FENa >0.5%; and 6) urine sodium >30 mEq/L [10]. In some cases, the diagnostic criteria include a serum ADH concentration greater than the measurement sensitivity [11]. However, an abnormal serum ADH concentration is not characteristic of SIADH alone and thus has no diagnostic value [12]. In this case, we investigated the possibility of both RSWS and SIADH as causes of hyponatremia. The patient had no apparent features of dehydration or renal dysfunction. Hyponatremia improved after treatment with water restriction and sodium correction. Therefore, the main reasons for the development of SIADH could have been excessive hydration to prevent renal dysfunction during cisplatin administration and the use of oral diuretics.
The usefulness of cisplatin/5-fluorouracil therapy as NAC for stage II and III advanced esophageal cancers has been previously reported [5]. In this case, cisplatin/5-fluorouracil therapy was administered as NAC and caused marked SIADH. Therefore, NAC was discontinued after 1 course and the patient underwent surgical treatment. Although chemotherapy was indicated for early postoperative recurrence, the risk of cisplatin-related SIADH recurrence was considered high. There is a case of SIADH after treatment with cisplatin in which the patient was able to continue treatment with carboplatin instead of cisplatin [13]. With regard to the available chemotherapy regimen for esophageal cancer, the PRODIGE5/ACCORD17 trial compared FOLFOX-radiotherapy (RT) and cisplatin/5-fluorouracil-RT therapy in patients diagnosed with stage I–IVA cancer and eligible for radical chemoradiotherapy and concluded that progression-free survival was similar with both regimens, but FOLFOX might be more convenient [3]. That case study also suggested that FOLFOX-RT may be an alternative to cisplatin/5-fluorouracil-RT. In addition, the E-DIS trial, performed for patients with unresectable or recurrent esophageal squamous cell carcinoma, showed comparable results between the FOLFOX and cisplatin/5-fluorouracil therapy [14]. In a study that compared the cisplatin/TS-1 and oxaliplatin/TS-1 therapy in patients with unresectable advanced or recurrent gastric cancer, 13.4% and 4.4% of patients in the cisplatin/TS-1 and oxaliplatin/TS-1 groups had hyponatremia, respectively [15]. Cisplatin-induced SIADH is considered to be caused by impaired electrolyte re-absorption at Henle’s loop and water overload due to large amounts of hydration [8], and it seems to occur less frequently with oxaliplatin since it does not require large amounts of hydration. However, even when using oxaliplatin, careful attention should be paid, as there have been reports of SIADH even with this regimen [11]. 5-fluorouracil and platinum-based regimens were considered preferable in this case because of the response to the preoperative cisplatin/5-fluorouracil therapy. Switching from cisplatin/5-fluorouracil therapy with cisplatin to FOLFOX therapy with oxaliplatin allowed our patient to continue treatment without subsequent development of SIADH. Oxaliplatin has several advantages over cisplatin, including that it does not require large amounts of hydration and can be performed on an outpatient basis. However, there is currently little evidence regarding the treatment of esophageal cancer, and great caution is required when using this drug.
Conclusions
The cisplatin/5-fluorouracil therapy is currently the standard chemotherapy regimen for esophageal cancer; however, SIADH is a known adverse effect when using cisplatin, as seen in the present case. To the best of our knowledge, there is no report of SIADH caused by cisplatin/5-fluorouracil therapy wherein the patient could continue chemotherapy without SIADH by switching to FOLFOX therapy with oxaliplatin. Oxaliplatin appears to have a lower risk of SIADH than cisplatin, suggesting that oxaliplatin can be a therapeutic option for patients with esophageal cancer who are at high risk of SIADH.
Figures
References:
1.. Lizuka T, Kakegawa T, Ide H, Phase Ⅱ evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: A Japanese Esophageal Oncology Group Trial: Jpn J Clin Oncol, 1992; 22; 172-76
2.. Kato K, Muro K, Ando N, A phase Ⅱ study of nedaplatin and 5-fluorouracil in metastatic squamous cell carcinoma of the esophagus: The Japan Clinical Oncology Group (JCOG) Trial (JCOG9905-DI): Esophagus, 2014; 11(3); 183-88
3.. Conroy T, Galais MP, Raoul JL, Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomized, phase 2/3 trial: Lancet Oncol, 2014; 15; 305-14
4.. Levin L, Sealy R, Barron J, Syndrome of inappropriate antidiuretic hormone secretion following dis-dichlorodiammineplatinum II in a patient with malignant thymoma: Cancer, 1982; 50; 2279-82
5.. Igaki H, Kato H, Ando N, A randomized trial of postoperative adjuvant chemotherapy versus neoadjuvant chemotherapy with cisplatin plus 5-fluorouracil for clinical stage II/III squamous cell carcinoma of the thoracic esophagus (JCOG 9907): J Clin Oncol, 2008; 26; 4510
6.. Berghmans T, Paemans M, Body JJ, A prospective study on hyponatraemia in medical cancer patients: Epidemiology, aetiology and differential diagnosis: Support Care Cancer, 2000; 8; 192-97
7.. Berghmans T, Hyponatremia related to medical anticancer treatment: Support Care Cancer, 2000; 8; 192-97
8.. Kurtzberg J, Dennis VW, Kinney TR, Cisplatinum-induced renal salt wasting: Med Pediatr Oncol, 1984; 12; 150-54
9.. Schwartz WB, Bennett W, Curelop S, Bartter FC, A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone: Am J Med, 2001; 12; 2860-70
10.. Decaux G, Musch W, Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone: Clin J Am Soc Nephrol, 2008; 3; 1175-84
11.. Goto Y, Wakita S, Yoshimitsu M, [Onset of syndrome of inappropriate secretion of antidiuretic hormone in a gastric cancer patient on SOX Treatment.]: Gan To Kagaku Ryoho, 2015; 42(13); 2467-70 [in Japanese]
12.. Melmed S, Jameson J: Endocrinology and metabolism. in: Harrison’s Principles of Internal Medicine, 2011; 2908-11, New York, McGraw-Hill
13.. Kagawa K, Fujitaka K, Isobe T, Syndrome of inappropriate secretion of ADH (SIADH) following cisplatin administration in a pulmonary adenocarcinoma patient with a malignant pleural effusion: Intern Med, 2001; 40; 1020-23
14.. Adenis A, Bennouna J, Etienne PL, Continuation versus discontinuation of first-line chemotherapy in patients with metastatic squamous cell oesophageal cancer: A randomized phase II trial (E-DIS): Eur J Cancer, 2019; 111; 12-20
15.. Yamada Y, Higuchi K, Nishikawa K, Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer: Ann Oncol, 2015; 26; 141-48
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250