15 June 2022: Articles 
A 28-Year-Old Woman Presenting with a Clinical Flare of Systematic Lupus Erythematosus and Abdominal Pain Due to Rectus Sheath Hematoma
Rare coexistence of disease or pathology
Jin-Lan Liao12ABDEF, Feng-Yan Zha13BCDEF, Brendan Smyth2DEF, Zu-Ying Xiong1DEF*DOI: 10.12659/AJCR.935472
Am J Case Rep 2022; 23:e935472
Abstract
BACKGROUND: A flare, or flare-up, of systematic lupus erythematosus (SLE) is diagnosed by an increase in disease activity in one or more organs, new symptoms, or changes in laboratory measurements. A hematoma can occur in the sheath of the rectus abdominis following muscle trauma or rupture of an epigastric vessel, or it can occur spontaneously. This report is of a 28-year-old woman who presented with a clinical flare of SLE and abdominal pain due to rectus sheath hematoma.
CASE REPORT: A 28-year-old woman had been suspected of having SLE 9 years ago and had received glucocorticoid therapy combined with hydroxychloroquine. However, lupus flared after she discontinued glucocorticoids, and she was admitted with a 1-month history of marked generalized edema, abdominal distension, frothy urine, and massive ascites. During hospitalization, she abruptly developed a continuous, stabbing abdominal pain and a bulge over the right abdomen as a result of straining during a bowel movement. On examination, a well-demarcated round mass that measured 121 mm × 96 mm was detected in the right quadrant. Abdominal emergency computed tomography revealed a right rectus sheath hematoma (21.4×4.7 cm). After her condition improved, the patient underwent an ultrasound-guided renal biopsy and was diagnosed with class III (A/C) and class V lupus nephritis.
CONCLUSIONS: This case has shown that spontaneous rectus sheath hematoma can occur without a history of trauma in a patient with an exacerbation of SLE. This association appears to be rare, and the cause is unknown.
Keywords: Hematoma, Lupus Erythematosus, Systemic, lupus nephritis, Abdominal Pain, Adult, Female, Humans, Muscular Diseases, Rectus Abdominis
Background
Systemic lupus erythematous (SLE) is a multisystemic disease with protean manifestations [1]. SLE flares are common in the disease course and can lead to permanent organ damage [2]. Although this disease is difficult to grasp and define, the new European League Against Rheumatism/American College of Rheumatology classification criteria for SLE added antinuclear antibodies to the entry criterion and provide an improved diagnosis of combined sensitivity and specificity [3]. Treatment of SLE aims at remission or low disease activity and prevention of flares [1]. Lupus nephritis influences the outcomes of SLE and guides the treatment and prognosis by its histologic classification, especially the 2003 revised International Society of Nephrology-Renal Pathology Society (ISN/RPS) classification, which is more advanced in predicting renal outcome [4].
Rectus sheath hematoma (RSH) is another rare clinical entity but has been previously reported in a patient with SLE [5]. RSH is characterized by accumulation of blood within the rectus sheath caused by disruption of the superior epigastric arteries, inferior epigastric arteries, and their branches or by direct damage to the abdominal wall [6]. Although it has been detected in only less than 2% of patients who present with acute abdominal pain, the overall mortality rate has been estimated at about 4% [6]. The mortality rate can be as high as 25% in patients on anticoagulation treatment and is 2 to 3 times higher in pregnant women [7]. Therefore, it is important to promptly identify RSH and make appropriate management.
This report is of a 28-year-old woman who presented with a clinical flare of SLE and abdominal pain due to RSH.
Case Report
A 28-year-old woman presented with photosensitivity, leukopenia, and positive antinuclear antibodies in 2013 and was suspected of having SLE, according to the 1982 American College of Rheumatology classification criteria for SLE. She received glucocorticoid therapy combined with hydroxychloroquine. Her initial daily dose of glucocorticoid was 25 mg of prednisone, which was tapered to a maintenance dose of 15 mg. The woman had not undergone renal biopsy and did not have a family history of SLE. She did have a history of cesarean delivery and appendectomy conducted in 2008 and 2010, respectively. She also reported noncompliance with the glucocorticoid regimen and had discontinued the use of oral prednisone and hydroxychloroquine since June 2016 because she had experienced acid reflux and indigestion. She refused to undergo regular treatment and was lost to follow-up until she consulted the hospital for severe edema in 2017 and was admitted to the Department of Nephrology in September 2017 with a 1-month history of marked generalized edema, abdominal dissension, frothy urine, cough, and slight dyspnea, without oral ulcer or arthritis.
Physical examination showed that her temperature was 36.5°C; respiratory rate, 19 breaths/min; oxygen saturation, 99% in room air; blood pressure, 153/82 mmHg; and weight, 77 kg. She had gained about 13.5 kg body weight within the span of 1 month. She had no rash or lymphadenopathy. Cardiopulmonary examination revealed hydrothorax. Shifting dullness was indicative of ascites, and her abdomen resembled that of a late-term gravid woman. Additionally, severe pitting edema was observed on both legs.
Laboratory tests (Table 1) were positive for the autoantibody ANA and related antibodies but negative for anti-dsDNA antibody. Hematuria, cylindruria, pyuria, and massive proteinuria (15.02 g/24 for 24-h urinary protein excretion) were also noted from the urinalysis. Pulmonary computed tomography (CT) examination in the outpatient department indicated pulmonary infection, and ceftriaxone was given.
A renal biopsy was not attempted due to severe edema and orthopnea. Treatment for a presumptive diagnosis of membrane lupus nephritis was commenced and initially comprised intravenous (i.v.) methylprednisolone (48 mg on days 1–15 of hospitalization) and i.v. immunoglobulin (on days 7–9) to block the autoantibody. Hemodialysis with heparin was done on day 6, as acute left heart failure was not relieved with the administration of furosemide and torasemide. On day 10 of admission, she abruptly developed a continuous, stabbing abdominal pain and a bulge over the right abdomen as a result of straining during a bowel movement. On examination, a well-demarcated round mass that measured 121×96 mm was detected in the right quadrant, without any redness, bruising, pulsation, or ecchymoses. An abdominal radiograph showed that the bowel appeared normal, without dilatation or excess air-fluid levels, and her serum lipase and amylase levels were within the reference range. Emergency CT of the abdomen (without administration of i.v. contrast) was performed. The image revealed a right RSH, massive ascites, and edema of the abdominal and intestinal walls (Figure 1); type 2 hematoma was classified in line with the bleeding between the fascia transversalis and the muscle from the CT scan [5]. There was no indication for an operation of the hematoma, given that her blood pressure, heart rate, and hemoglobin level were stable.
She experienced acute kidney injury on day 13 as loss of blood caused by RSH. At this point, the SLE disease activity index of the patient was 19 (based on proteinuria, hematuria, cylindruria, pyuria, leukopenia, and pleurisy). A persistent lupus flare was diagnosed, and she was treated with a pulse of 500 mg of i.v. methylprednisolone daily for 3 consecutive days (days 16–18) followed by 80 mg of i.v. methylprednisolone daily for the next month. Additionally, 600 mg of i.v. cyclophosphamide was administered on day 12, followed by 400 mg administered every 2 weeks. She also experienced relief from neuropsychiatric systemic lupus erythematosus (NPSLE) on day 22, which manifested with visual hallucination and persecutory delusion and was relieved on day 25 by plasmapheresis and i.v. injection of immunoglobulin.
After 6 weeks of treatment, her inflammatory marker levels had improved, and her abdominal pain was reduced to mild levels. A follow-up CT scan (Figure 2) taken at day 51 indicated a reduction that was more than 50% in size of the right RSH, and there was no need for a further procedure. The daily dose of i.v. methylprednisolone was reduced from 80 mg to 24 mg at the rate of 8 mg per week, and oral cyclosporine treatment was commenced (50 mg twice a day) from day 49. The total cyclophosphamide dose reached 2.2 g over the 2-month hospitalization period. Puncture and drainage of ascites were performed on day 75 after her condition improved. The drained fluid was turbid and milky white in appearance and had a volume of 800 mL, and it was positive for the chylous test and Rivalta test, with a protein concentration of 3.6 g/L and a lac-tate dehydrogenase level of 40 U/L. The nucleated cell count was 218×106/L, with 72% monocytes. The massive chylous ascites gradually disappeared during 1 month of drainage. The patient underwent an ultrasound-guided renal biopsy on day 76. Of the 14 glomerulus samples obtained, 1 was sclerotic and 1 showed segmental sclerosis. The glomeruli displayed mild to moderate segmental mesangial hypercellularity and matrix proliferation, with thickening of the glomerular basement membrane (Figure 3). The thickness of the glomerular basement membrane was 1500 nm, as demonstrated by electron microscopy. Immunofluorescence analysis revealed a full-house pattern (Figure 4). The patient was diagnosed with class III (A/C) and class V lupus nephritis according ISN/RPS 2003 classification [8] and was discharged on day 110 after admission; she was given a prescription of methylpredniso-lone and cyclosporine (Figure 5). On day 216, her urine protein level had fallen to 1.13 g/day (Table 1).
We have received the informed consent of the patient for the publication of this report.
Discussion
Patients with SLE often experience disease flares of varying severity [9]. Prompt and accurate SLE flare assessment is central in routine clinical practice [2]. The present paper reports a rare onset case of RSH associated with SLE flare, in light of proteinuria, hematuria, cylindruria, pyuria, leukopenia, pleurisy, and NPSLE in the course of the disease. Although risk factors for RSH such as anticoagulant use and history of prior surgery exist in this case, we propose that the massive ascites contributed to increasing intra-abdominal pressure in the setting of the SLE flare and thus to the development of RSH.
The mechanism of RSH is not completely understood and is believed to be caused by several factors. Anatomy plays a significant role in the formation of RSH. The posterior surface of the rectus abdominis muscle under the arcuate line is covered only by the relatively weak transversalis fascia and the peritoneum [10], and the inferior epigastric arteries are more likely to be injured under the arcuate line without the protection of the rectus sheath. Other risk factors include direct abdominal trauma, previous abdominal surgery, the use of anticoagulation therapy, coughing or intense rectus muscle contraction, pregnancy, and infection [5–7,11].
Tutak et al first reported a grade III spontaneous RSH (10×16 cm) concomitant with SLE in a 78-year-old man and used a low dose of anticoagulant for thromboembolism prophylaxis briefly; however, SLE was considered responsible for causing the bleed [5]. Our case was a 28-year old woman with an SLE flare involved in major organs, including cytopenias, neuropsychiatric lupus, and nephritis. The serum C3 was lower than in the previously reported case and anti-double stranded DNA (anti-dsDNA) was negative. The anti-dsDNA negative status seems to identify another subset of patients with peculiar clinical features [12], in particular, serositis. The risks associated with disease flares were withdrawal of maintenance immunosuppressive treatment and discontinuation of hydroxycholoroquine [9]. On the CT scan of our patient, RSH was more giant (21.4×4.7 cm) than the previously reported case and was designated as type II hematoma because it was within the rectus muscle and between the muscle and transversalis fascia [13]. Surgery was not considered in either case, as hemodynamics were not impaired. In the present case, multiple factors may have contributed to RSH. First, the SLE flare may have increased susceptibility, as it is a state associated with complement activation, circulating immune complex formation, in situ immune complex deposition, vascular injury, and, eventually, increased capillary permeability [10]. Second, the patient had been receiving anticoagulation therapy for both venous thromboembolism prophylaxis and intradialytic anticoagulation. Third, as is common during a flare of SLE [14], her platelet count was mildly reduced. Fourth, the patient had severe hypoproteinemia and massive ascites that could lead to increased abdominal pressure and susceptibility to RSH. Ascites is rare in SLE, even rarer in the chylous form. The mechanism of chylous as-cites in SLE remains unknown, but one may speculate about lymphatic stenosis or an increase in endoluminal pressure and permeability of vascular walls [15]. Other potential causes of chylous ascites, including tuberculosis, filariasis, liver cirrhosis, neoplastic and traumatic factors, were excluded [16]. Lupus peritonitis was also excluded because of a serum-ascites albumin gradient greater than 1.1 [17]. Finally, coughing and difficultly in defecation were probably the causes in the present RSH [13,18]. This case highlights the importance of simple measures, such as aperients, to reduce the need for abdominal straining in patients with marked ascites, hypoproteinemia, and an inflammatory state.
Management of RSH depends on the patient’s clinical status and the underlying cause [6]. Patients who are hemodynamically stable without any changes in their serum hemoglobin are usually closely observed and treated with analgesics if they experience pain [13]. Additionally, anticoagulation therapy is temporarily discontinued, when appropriate [13]. Attention to ensuring regular and soft consistency of stool is also important to avoid the risk of further bleeding [19]. A supportive blood transfusion may be indicated and, in rare cases, angioembolization of the bleeding vessel may be required for patients who are in hemorrhagic shock or have a marked decrease in hemoglobin and ongoing bleeding [18]. In our case, the control of the SLE flare with glucocorticoids and immunosuppressants was important, as this alleviated the inflammatory syndrome and associated metabolic derangements that may have contributed to this complication.
Conclusions
This case has shown that spontaneous RSH can occur without a history of trauma in a patient with an exacerbation of SLE. This association appears to be rare, and the cause is unknown.
Figures
Tables
Table 1.. Laboratory data.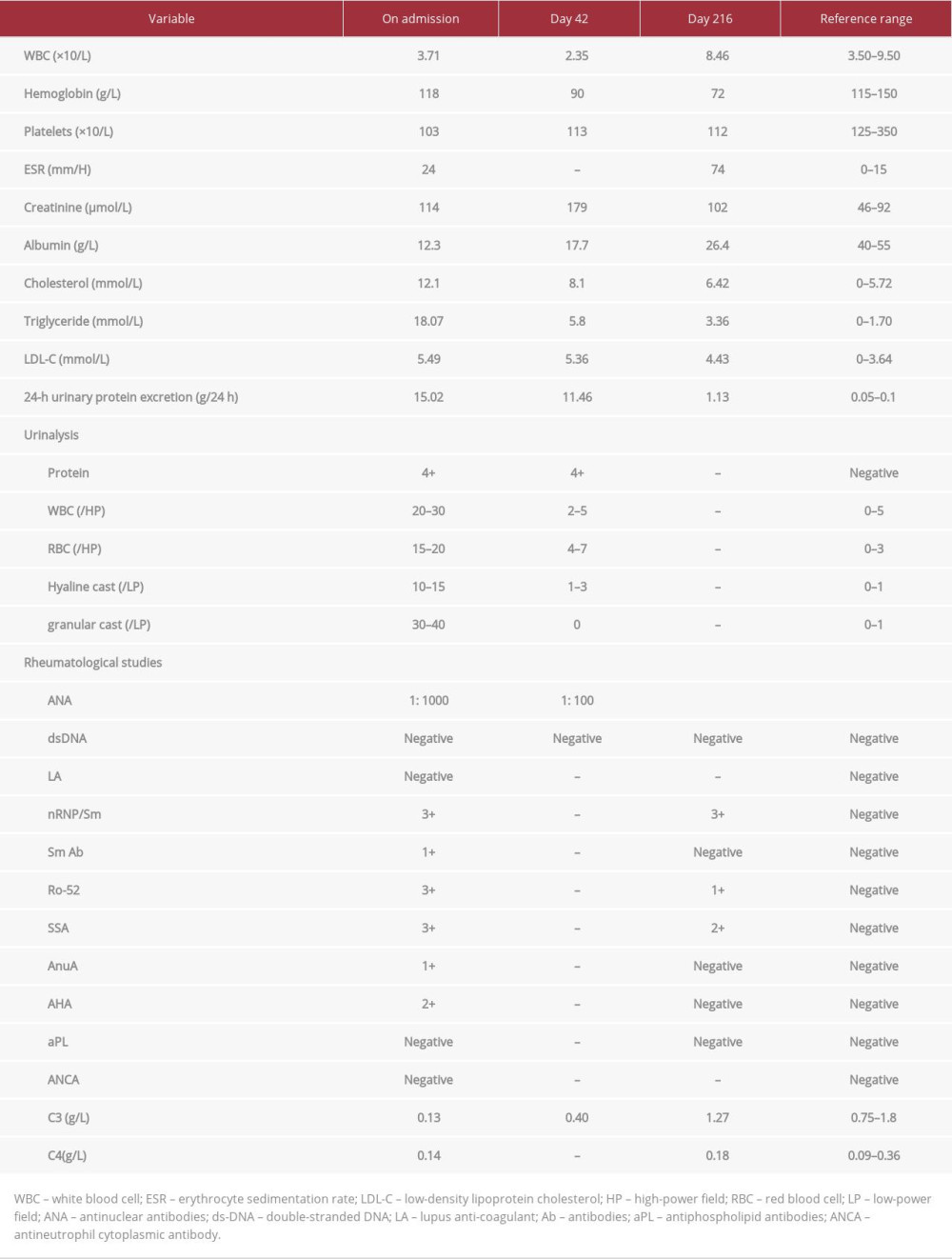
References:
1.. Fanouriakis A, Kostopoulou M, Alunno A, 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus: Ann Rheum Dis, 2019; 78(6); 736-45
2.. Thanou A, Jupe E, Purushothaman M, Clinical disease activity and flare in SLE: Current concepts and novel biomarkers: J Autoimmun, 2021; 119; 102615
3.. Aringer M, Costenbader K, Daikh D, 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus: Arthritis Rheumatol, 2019; 71(9); 1400-12
4.. Hwang J, Kim HJ, Oh J-M, Outcome of reclassification of World Health Organization (WHO) class III under International Society of Nephrology-Renal Pathology Society (ISN-RPS) classification: retrospective observational study: Rheumatol Int, 2012; 32(7); 1877-84
5.. Tutak AS, Findikli HA, Aslan S, Tastekin E, Grade III spontaneous rectus sheath hematoma concomitant to SLE: Archives of Clinical and Medical Case Reports, 2018; 2(5); 162-67
6.. Hatjipetrou A, Anyfantakis D, Kastanakis M, Rectus sheath hematoma: Aa review of the literature: Int J Surg, 2015; 13; 267-71
7.. Agarwal S, Lamani YP, Goudar BV, Rectus sheath haematoma secondary to enoxaparin injection – a rare case report: J Clin Diagn Res, 2017; 11(3); PD11-12
8.. Weening JJ, D’agati VD, Schwartz MM, The classification of glomerulonephritis in systemic lupus erythematosus revisited: Kidney Int, 2004; 65(2); 521-30
9.. Adamichou C, Bertsias G, Flares in systemic lupus erythematosus: diagnosis, risk factors and preventive strategies: Mediterr J Rheumatol, 2017; 28(1); 4-12
10.. Santacruz JC, Mantilla MJ, Rueda I, A practical perspective of the hematologic manifestations of systemic lupus erythematosus: Cureus, 2022; 14(3); e22938
11.. Ünlüer EE, Kaykısız EK, An unanticipated diagnosis with bedside ultrasonography in patients with acute abdominal pain: rectus hematoma: Pan Afr Med J, 2017; 27(1); 19
12.. Fabrizio C, Fulvia C, Carlo P, Systemic lupus erythematosus with and without anti-dsDNA antibodies: analysis from a large monocentric cohort: Mediators Inflamm, 2015; 2015; 325078
13.. Allen M, Sevensma KE, Rectus sheath hematoma: StatPearls, 2022, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.
14.. Keeling DM, Isenberg DA, Haematological manifestations of systemic lupus erythematosus: Blood Rev, 1993; 7(4); 199-207
15.. Zhang G-H, Zhang L-L, Wang Y-H, Shen W-B, Clinical characteristics of systemic lupus erythematosus with chylothorax and/or chylous ascites: An analysis of 15 cases in China: Medicine (Baltimore), 2020; 99(51); e23661
16.. Bhardwaj R, Vaziri H, Gautam A, Chylous ascites: A review of pathogenesis, diagnosis and treatment: Journal of clinical and translational hepatology, 2018; 6(1); 105
17.. Cook SG, Ascites as the presenting sign of systemic lupus erythematosus: Cureus, 2022; 16(3); e23131
18.. Kasotakis G, Retroperitoneal and rectus sheath hematomas: Surg Clin North Am, 2014; 94(1); 71-76
19.. Patel J, Hameed R, Joseph L, Constipation-induced spontaneous rectus sheath hematoma: 1908: Am J Gastroenterol, 2017; 112; S1054
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








