15 August 2022: Articles 
Degenerated Serous Cystic Tumor of the Pancreas: Case Report and Literature Review of an Aggressive Presentation of a Benign Tumor
Challenging differential diagnosis, Rare coexistence of disease or pathology
Xenofon Papazarkadas1ABCDEF, Eleftherios Gialamas2ABCDEF*, Galab M. Hassan3AE, Roland Chautems1BF, Aurelie Bornand4AEF, Giacomo Puppa4AF, Christian TosoDOI: 10.12659/AJCR.936165
Am J Case Rep 2022; 23:e936165
Abstract
BACKGROUND: Serous cystic tumors of the pancreas are known to present a benign nature and course, not requiring surgery in the absence of symptoms. In rare cases, these benign tumors may present aggressive characteristics such as local infiltration and lymph node and distant metastases. In such cases, a surgical approach may be necessary.
CASE REPORT: We present the case of a 79-year-old woman with an asymptomatic cytologically suggested caudal serous cystic tumor infiltrating the spleen and the splenic vein. This tumor was discovered in a computed tomography scan in the setting of evaluating distant spreading of a primary malignant neoplasm of the rectum. Suspicious malignant signs on imaging dictated a surgical approach and a distal splenopancreatectomy was carried out in the same operative time as the transanal resection of the rectal lesion. The nature of the pancreatic neoplasm was confirmed by histology, but 2 lymph nodes out of 4 retrieved were positive. The postoperative course was uneventful. No adjuvant treatment was proposed. Imaging control 6 months after surgery was not indicative of relapse.
CONCLUSIONS: Serous cystic adenomas of the pancreas, although generally considered benign neoplasms, may present with characteristics of malignancy. Moreover, they may prove difficult to differentiate from other malignant neoplasms by non-surgical modalities. Although current guidelines and data from the literature provide controversial information regarding management of these clinical entities, in the presence of suspicious radiological aspects, surgical resection could be considered.
Keywords: Cystadenocarcinoma, Serous, Cystadenoma, Serous, Pancreatic Cyst, Abdomen, Aged, Female, Humans, Neoplasm Recurrence, Local, Pancreas, Pancreatic Neoplasms, Tomography, X-Ray Computed
Background
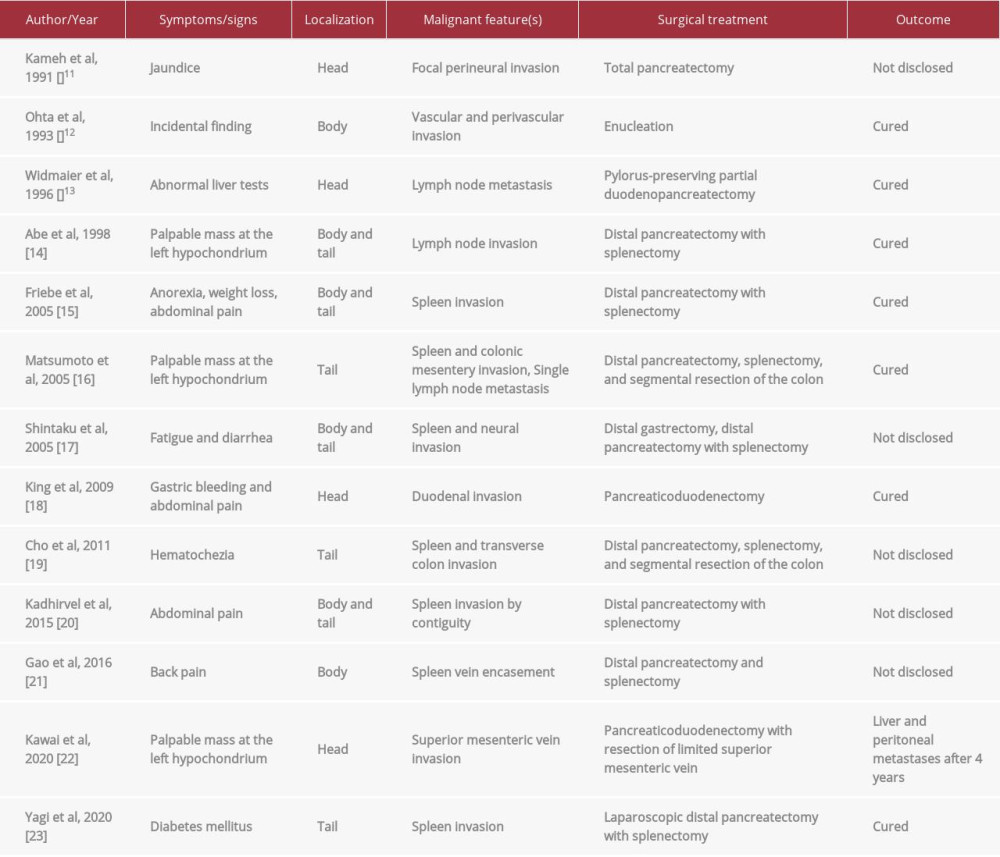
Serous cystic tumor of the pancreas is a rare clinical entity falling into the category of pancreatic cystic neoplasms, along with mucinous cystic tumors and intraductal papillary mucinous neoplasms [1,2]. It can be sporadic or associated with Von-Hippel-Lindau disease. In contrast to its mucin-producing counterparts, malignancy of serous cystic tumors is exceedingly uncommon [1] and this type of tumor tends to be considered as a completely benign lesion. Nonetheless, serous cystic tumors with locally aggressive behavior have been observed. The scarce epidemiological data regarding locally invasive or metastasizing tumors make the proposition of an optimal treatment difficult. In the present study, we present a case of an asymptomatic serous cystic tumor with invasion of nearby tissues without metastasis.
Case Report
A 79-year-old female patient was diagnosed with a 4.2 cm low rectal polyp during screening colonoscopy, 13 cm from the anal verge, and transanal resection was planned. She was a non-smoker, with past light alcohol consumption and her medical history consisted of hypercholesterolemia and hiatal hernia, without family history of cancer. A subsequent computed tomography (CT) scan in search of potential metastases showed a mass located at the tail of the pancreas measuring 12×6.8×9.8 cm with invasion of the hilum of the spleen and splenic vein thrombosis with segmental portal vein hypertension (Figure 1). Blood chromogranin A level was negative, as were tumor markers (CA 19.9 <9 kU/l, CEA<1.8 µg/l). A pancreatic magnetic resonance imaging (MRI) confirmed the presence of a mass at the tail of the pancreas, which is typically associated with a serous cystic adenoma. However, this mass showed invasion of the splenic parenchyma and splenic vein (Figure 2). A second, smaller lesion was found at the uncinate process, and was considered to be an intraductal papillary mucinous neoplasm. An endoscopic ultrasound with fine-needle aspiration was then performed, confirming the presence of these 2 lesions (one in the pancreatic body and the other in the pancreatic tail) with cytopathology of the caudal lesion showing serous cystic adenoma. After discussion in a multidisciplinary tumor board, surgical resection was proposed, based on the locally invasive characteristics. Distal splenopancreatectomy by laparotomy was performed, together with transanal excision of the rectal polyp (TAMIS). Operative time was 240 min and intraoperative blood loss was estimated at 250 ml. Postoperative course was uneventful and the patient was discharged at postoperative day 8.
Histopathology of the specimen showed a 12 cm serous micro-cystic tumor of the pancreatic tail without atypia, with a partially calcified fibrous stroma invading the spleen and splenic vein (Figure 3). Of the 4 retrieved lymph nodes, 2 were positive, 1 of which appeared to be infiltrated in continuity with the tumor. Whether the second lymph node was also an infiltration in continuity with the tumor or a lymphatic metastasis could not be specified. The second lesion at the pancreatic body was a mixed gastric subtype intraductal papillary mucinous neoplasm without signs of malignancy. Analysis of the rectal polyp showed moderately differentiated adenocarcinoma developing on a tubulovillous adenoma with low- and high-grade dysplasia with negative resection margins.
After a new multidisciplinary oncologic discussion, the pancreatic tumor was classified as a locally invading serous cystic tumor and no adjuvant treatment was proposed. Followup with a thoraco-abdominal CT scan at 6 months postsurgery showed no recurrence.
Discussion
Although serous cystic tumors are rare lesions, their prevalence remains unknown. One retrospective review of 24 039 patients undergoing CT scan and MRI showed a 0.7% combined incidence of serous cystic adenomas and mucinous cystic adenomas in the general population [3], while serous cystic adenomas may represent 10–16% of total pancreatic cystic neoplasms [4,5].
The macroscopic appearance of serous cystic adenomas may vary, with 4 typical types: microcystic (multiple cysts <2 cm), macrocystic (multiple cysts >2 cm), mixed type (variable-sized cysts) and solid (no visible cysts in imaging) [6], with micro-cystic being the most common morphology and solid being the rarest variant [7]. Microscopically, serous cystic adenomas demonstrate monomorphous cuboidal-shaped epithelium. Cells are glycogen-rich with cellular cytoplasm and small regular nuclei; mitotic activity is absent. Classification of these tumors as benign, borderline, or malignant depends on the cells’ nuclear features [6].
In general, serous cystic adenomas are considered benign lesions with almost nonexistent malignant degeneration. Larger lesions may sometimes infiltrate local lymph nodes or adjacent organs (spleen, stomach, colon) [1]. A noteworthy observation is that such neoplasms with the same monomorphous histo-logical appearance may present signs of malignancy such as invasion of nearby tissues (such as the spleen, splenic vein, or lymph nodes) or distant metastases. Serous cystadenocarcinoma is a term employed for these aggressively behaving variants. Khashab et al mention that histology of serous cystadenoma is insufficient to confirm malignancy, and it is therefore defined by the neoplasm’s invading or metastasizing behavior [8]. In 1996, the World Health Organization’s
The optimal management of serous cystic adenomas remains controversial to this day. Generally, watchful waiting is proposed, due to their benign nature, with surgical resection being reserved for symptomatic tumors or when malignancy cannot be formally excluded [10]. In the latter cases, oncological re-section with followup, as is done for pancreatic cancer, is recommended. Conversely, guidelines from the European Study Group on Cystic Tumors of the Pancreas completely discard the possibility of a malignant serous cystic adenoma and reserve operation only for symptomatic patients, proposing no followup for serous cystic lesions when diagnosis is certain [11]. In our case, radiological invasion of the spleen and splenic vein compelled the surgical resection of this potentially degenerated lesion.
Finally, to date, there are no cases describing the coexistence of rectal adenocarcinoma and serous cystic tumors of the pancreas. Data in the pertinent literature do not support a potential connection, via genetic predisposition, between the 2 neoplasms, although these data are scarce and dedicated studies do not exist [12,13].
Conclusions
A grey area exists between aggressively behaving serous cystic adenomas and serous cystic adenocarcinomas. Although rare entities, these tumors pose a medical riddle for the practitioner, posing the question of whether surgical resection only would suffice to avoid malignancy-associated sequalae like disease relapse or metachronous metastases. A unanimous agreement is needed to better define these entities and evaluate their malignant potential, which is the deciding factor for their management.
Figures
References:
1.. Nagtegaal ID, Odze RD, Klimstra D, The 2019 WHO classification of tumours of the digestive system: Histopathology, 2020; 76(2); 182-88
2.. Klöppel G, Solcia E, Longnecker DS: Histological typing of tumours of the exocrine pancreas, 1996, WHO
3.. Verbesey JE, Munson JL, Pancreatic cystic neoplasms: Surg Clin North Am, 2010; 90(2); 411-25
4.. Kosmahl M, Pauser U, Peters K, Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: A review of 418 cases and a classification proposal: Virchows Arch, 2004; 445(2); 168-78
5.. Valsangkar NP, Morales-Oyarvide V, Thayer SP, 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital: Surg (United States), 2012; 152(3 Suppl.); s4-12
6.. Parks R: Hepatobiliary and Pancreatic Surgery, 2019, Elsevier
7.. Compton CC, Histology of cystic tumors of the pancreas: Gastrointest Endosc Clin N Am, 2002; 12(4); 673-96
8.. Khashab MA, Shin EJ, Amateau S, Tumor size and location correlate with behavior of pancreatic serous cystic neoplasms: Am J Gastroenterol, 2011; 106(8); 1521-26
9.. Jais B, Rebours V, Malleo G, Serous cystic neoplasm of the pancreas: A multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas): Gut, 2016; 65(2); 305-12
10.. Del Chiaro M, Verbeke C, Salvia R, European experts consensus statement on cystic tumours of the pancreas: Dig Liver Dis, 2013; 45(9); 703-11
11.. Del Chiaro M, Besselink MG, Scholten L, European evidence-based guidelines on pancreatic cystic neoplasms: Gut, 2018; 67(5); 789-804
12.. Tseng JF, Warshaw AL, Sahani DV, Serous cystadenoma of the pancreas: Tumor growth rates and recommendations for treatment: Ann Surg, 2005; 242; 413-21
13.. Halder PJ, Sharma S, Nikhil S, A left-sided cystic pancreatic incidentaloma with sigmoid colon adenocarcinoma: A case report: J Med Case Rep, 2018; 12(1); 251
Figures
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250