08 June 2022: Articles 
Rhabdomyolysis and Acute Kidney Injury Associated with Infection: A Report of 2 Cases
Management of emergency care, Rare disease
In Hee Lee1ABCDEF*, Dong Jik Ahn2BCDFDOI: 10.12659/AJCR.936407
Am J Case Rep 2022; 23:e936407
Abstract
BACKGROUND: Rhabdomyolysis is a clinical syndrome characterized by elevated serum creatine kinase (CK) and myoglobin levels due to the breakdown of muscle fibers and is associated with symptoms such as myalgia, muscle swelling, and erythruria. Rhabdomyolysis has an array of potential causes, including Salmonella infection, although rare. We report 2 cases in which nontyphoidal salmonellae caused acute gastroenteritis complicated by rhabdomyolysis and myoglobinuric acute kidney injury (AKI).
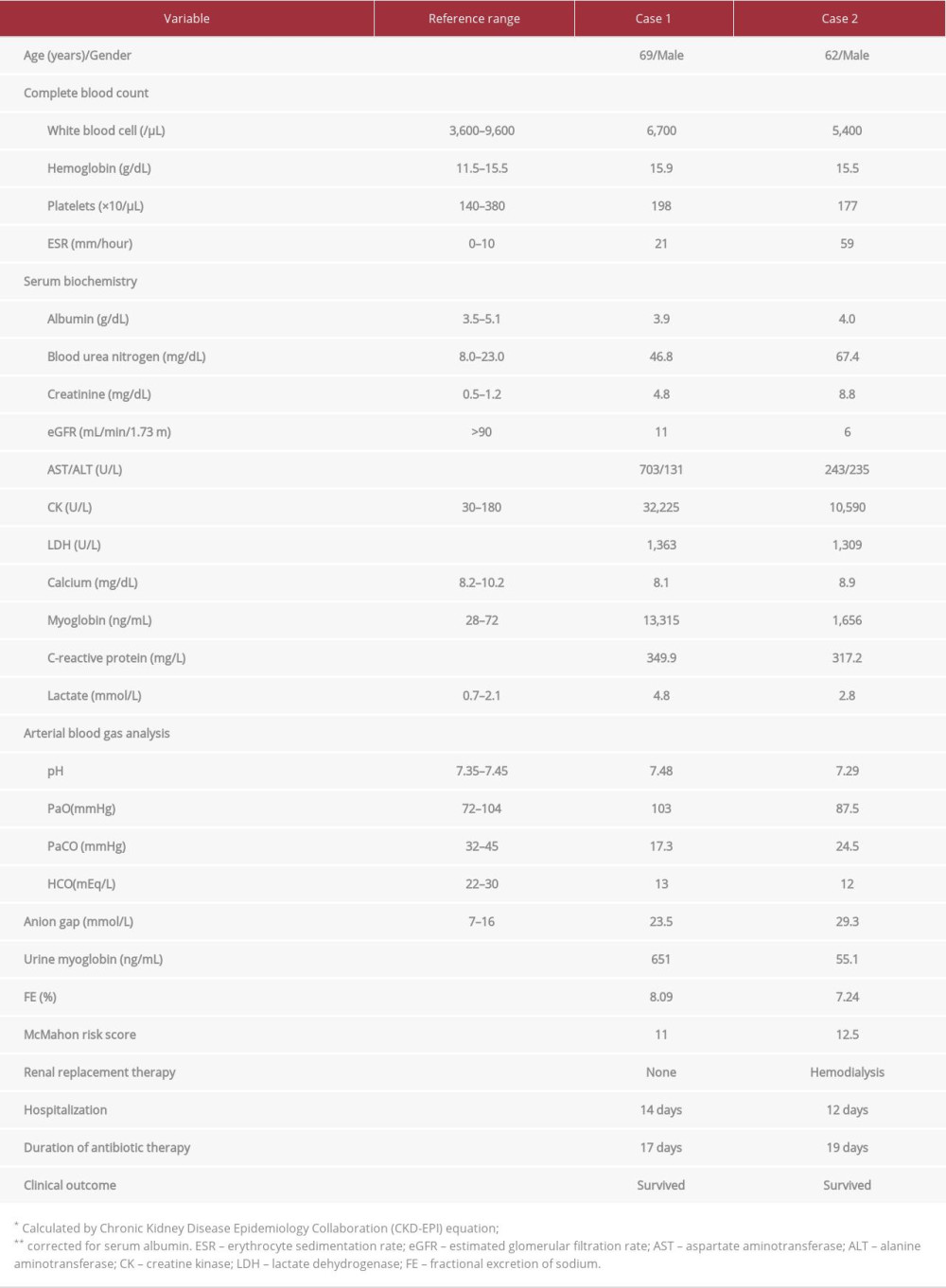
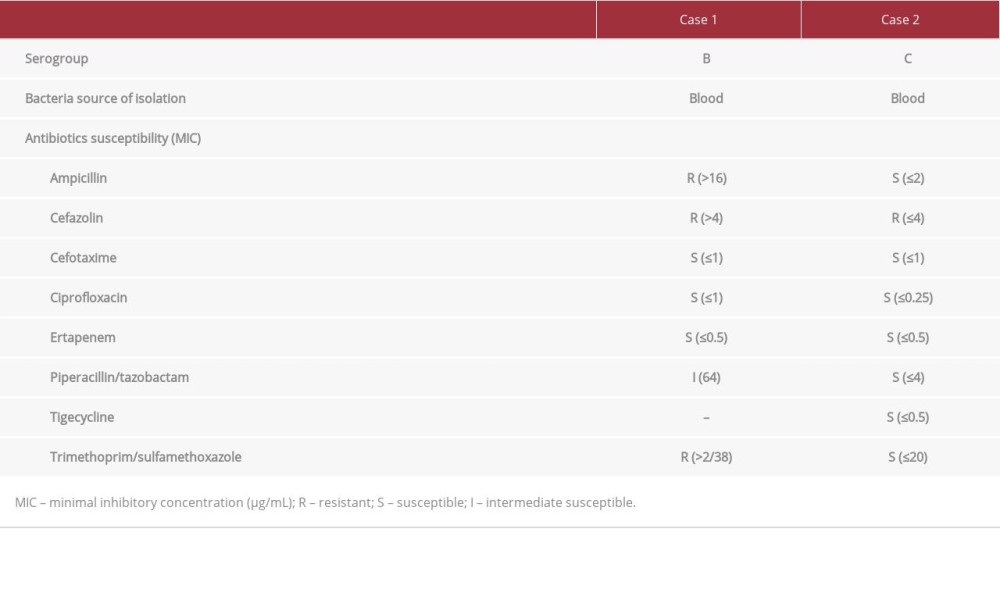
CASE REPORT: Two male patients, aged 69 years and 62 years, presented to our hospital with sudden-onset fever, abdominal pain, and watery diarrhea. At the time of admission, the patients had elevated serum CK levels (32 225 U/L and 10 590 U/L, respectively) and serum creatinine levels (4.8 mg/dL and 8.8 mg/dL, respectively). Both patients also had elevated serum myoglobin concentrations with significant myoglobinuria. They were administered fluid therapy and intravenous empirical antibiotics (cefotaxime and metronidazole for Case 1, ciprofloxacin for Case 2). The patient in Case 2 underwent 3 sessions of hemodialysis due to persistent oliguria and exacerbation of metabolic acidosis. Salmonella B (Case 1) and Salmonella C (Case 2) were isolated from blood cultures. After about 2 weeks of inpatient care, both patients showed improvement of clinical symptoms and were discharged.
CONCLUSIONS: Patients with acute gastroenteritis induced by Salmonella infection can develop rhabdomyolysis and myoglobinuric AKI in rare cases. Timely administration of appropriate antibiotics and fluids is expected to produce a favorable prognosis. Furthermore, early initiation of hemodialysis after onset of oliguric AKI can improve clinical outcomes.
Keywords: Acute Kidney Injury, rhabdomyolysis, Anti-Bacterial Agents, creatine kinase, Gastroenteritis, Humans, Male, Myoglobin, Salmonella Infections
Background
Rhabdomyolysis is a clinical syndrome characterized by muscle weakness, myalgia, muscle swelling, erythruria, and release of intracellular components (eg, potassium, phosphate, myoglobin, creatine kinase [CK], and uric acid) into the plasma as a result of damaged skeletal muscle fibers [2]. During the course of this disease, severe complications can occur, including acute kidney injury (AKI), hypovolemic shock, and arrhythmias [3]. The major causes of rhabdomyolysis include trauma, chronic alcoholism, seizures, electrolyte disorders, muscular dystrophies, infections, drug abuse, and exposure to toxins. Infection is the cause of rhabdomyolysis in approximately 5% of cases [4]. Infection-associated rhabdomyolysis is generally caused by viral diseases, including influenza and HIV infection, as well as fungal and protozoal infections. Rhabdomyolysis is relatively rarely caused by bacterial infections, such as
Case Reports
CASE 1:
A 69-year-old man presented to the Emergency Department (ED) with high fever and chills that had persisted for 2 days. He had consumed beef sashimi 5 days before admission and developed diffuse abdominal pain and watery diarrhea the following day. He had been taking an angiotensin converting enzyme inhibitor (olmesartan 40 mg/day) for hypertension that was diagnosed 10 years prior. Two months earlier, his serum creatinine (Cr) level had been 1.1 mg/dL. He had no other chronic diseases such as diabetes mellitus or liver cirrhosis. At the time of admission, his vital signs were blood pressure, 110/70 mmHg; pulse, 117 beats/min; respiratory rate, 20 breaths/min; and body temperature, 37.7°C. Physical examination revealed a rigid abdomen without rebound tenderness. In addition to azotemia, the laboratory findings at the time of admission showed elevated serum levels of muscle enzymes, namely CK, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and alanine aminotransferase, as well as an elevated C-reactive protein (CRP) level (Table 1). The patient’s urine was dark-brown, and routine urinalysis showed a pH of 5.5, protein±, and occult blood 3+, and microscopic urinalysis showed 1–4 white blood cells (WBCs) per high power field (HPF) and 3–9 red blood cells (RBCs) per HPF. The urine was also positive for myoglobin. Serologic test results were negative for hepatitis B surface antigen (HBs Ag), anti-hepatitis A virus (anti-HAV) IgM, anti-hepatitis C virus antibody (anti-HCV Ab), anti-HIV Ab, and influenza A, B. Abdominal ultrasonography revealed normal echogenicity in both kidneys, with no findings suggestive of chronic kidney disease or obstructive uropathy.
Following patient admission, we began intravenous (i.v.) administration of isotonic saline (200-300 mL/hour) and a vasopressor. Empirical antibiotic therapy was initiated with cefotaxime (2 g daily) and metronidazole (500 mg 4 times daily). On hospitalization day 2, i.v. fluids and diuretics were administered to maintain urine output (>100 mL/h, 1.5 mL/kg/h). However, the laboratory findings worsened, with the serum Cr level reaching 5.4 mg/dL, CK 33 697 U/L, and AST 801 U/L. On hospitalization day 4, the patient’s stool culture was negative for pathogenic bacteria; however, growth of Salmonella B was confirmed on blood culture (Table 2). Technetium-99m hydroxymethylene diphosphonate (99mTc-HDP) bone scintigraphy obtained on hospitalization day 10 revealed diffusely increased isotope uptake in both shoulders, upper arms, and hip areas (Figure 1). On hospitalization day 14, the serum Cr concentration, estimated glomerular filtration rate (eGFR), and CK level had returned to normal (0.7 mg/dL, 96 mL/min/1.73 m2, and 65 U/L, respectively), and repeated blood and feces cultures were negative; thus, the patient was discharged. At 3 months after hospital discharge, the patient showed serum blood urea nitrogen (BUN) of 19.4 mg/dL and serum Cr of 1.0 mg/dL.
CASE 2:
A 62-year-old man was transferred to our ED with fever and oliguria that had persisted for 2 days. The patient had eaten raw beef liver 5 days earlier and had diffuse abdominal pain and watery diarrhea (5 to 6 episodes per day) starting 3 days earlier. He was given medical treatment, including fluid therapy and i.v. antibiotics, at a secondary care facility; however, the oliguria persisted (urine output <100 mL/day). He had been taking calcium channel blockers (nifedipine, 60 mg/day) since hypertension had been diagnosed 15 years earlier. The patient’s vital signs at admission included a blood pressure of 100/70 mmHg, pulse rate of 88 beats/min, respiratory rate of 20 breaths/min, and body temperature of 38.3°C. The peripheral blood examination and biochemical test results upon admission are presented in Table 1. Viral serology tests were negative for HBs Ag, anti-HAV IgM, anti-HCV Ab, anti-HIV Ab, and influenza A, B. The patient’s urine was reddish brown, and routine urinalysis showed a pH of 5.5, protein 2+, and occult blood 3+. Microscopic examination of urine sediments showed 10–29 WBCs per HPF and 3-9 RBCs per HPF.
Upon arrival at the ED, the patient was immediately administered i.v. isotonic saline (0.9% NaCl, 300–500 mL/h) and i.v. ciprofloxacin (400 mg/day). Because of persistent oliguria and exacerbation of pulmonary edema and metabolic acidosis (arterial blood pH 7.02), even after continuous i.v. administration of loop diuretics (furosemide 20 mg/h), emergent hemodialysis (2 h) was performed 8 h after admission. On hospitalization day 2, clinical anuria (urine output <100 mL/24 h) persisted even with i.v. diuretics; therefore, a second session of hemodialysis (4 h) was implemented. The patient was maintained on fluid therapy with isotonic NaCl plus 5% glucose solution (150 mL/h). On hospitalization day 4, the blood culture result was positive for Salmonella C (Table 2). A third hemodialysis (4 h) was performed on the same day. On hospitalization day 7, daily urine output increased to 1 L or more, and serum CK and myoglobin levels decreased to 4455 U/L and 391 ng/mL, respectively. A 99mTc-HDP bone scan performed on the same day showed increased isotope uptake in the soft tissue of the both arms and legs (Figure 2). On hospitalization day 12, the patient’s renal function had returned to normal (serum Cr, 0.8 mg/dL; eGFR 95 mL/min/1.73 m2). Serum levels of muscle enzyme were within the reference ranges, and the gastrointestinal discomfort was markedly resolved; therefore, the patient was discharged.
At 3 months after hospital discharge, his renal function was normal, with a serum Cr level of 0.8 mg/dL.
Discussion
The clinical manifestations of rhabdomyolysis vary widely depending on degree and severity of muscle injury, from asymptomatic serum elevations of muscle enzymes (CK, LDH, or AST) to severe muscle pain, swelling, weakness, and reddish-brown urine. Furthermore, patients can show reduced intravascular fluid volume, metabolic acidosis, and various electrolyte imbalances (eg, hyperkalemia, hyperphosphatemia, and hypocalcemia). Rhabdomyolysis is diagnosed based on musculoskeletal symptoms, with an increase of serum CK to ≥1000 U/L or 5 to 10 times higher than the upper reference limit and laboratory findings including significant myoglobinuria [3]. The mechanism by which
Approximately 10% to 40% of rhabdomyolysis cases are complicated by AKI, which increases morbidity and mortality rates. The reported etiologies of AKI include reduced blood flow, acute tubular necrosis and obstruction caused by myoglobin, renal vasoconstriction, and free radical production [11]. In a study that analyzed 60 cases of infection-associated rhabdomyolysis, the proportion of
In both cases, 99mTc-HDP bone scintigraphy was performed during hospitalization. Although bone scintigraphy has lower spatial resolution compared to magnetic resonance imaging in patients with rhabdomyolysis, it is useful for evaluating the site and extent of muscle injury through systemic survey [14,15]. Moreover, it has been reported that bone scintigraphy can be helpful in estimating the cause of rhabdomyolysis according to the intensity and distribution pattern of soft tissue involvement [16]. Disruption of myocyte membrane and subsequent mitochondrial chelation has been suggested as a potential mechanism for the accumulation of phosphate-containing radiotracers in the damaged muscles [15]. The diffuse and symmetric pattern of increased radioisotope uptake in the bone scans of our 2 patients can be regarded as radiologic findings suggestive of non-traumatic rhabdomyolysis (such as infection-associated) rather than post-traumatic rhabdomyolysis [16].
Fractional excretion of sodium (FENa) is a useful urinary index for determining the functional integrity of renal tubules. Patients with rhabdomyolysis and myoglobinuric AKI have a low FENa (<1%) due to factors such as volume depletion, preglomerular vasoconstriction, and tubular occlusion [11]. Our 2 patients had no history of chronic kidney disease or prior use of diuretics; however, their FENa was elevated by more than 2% at the time of admission (8.09% and 7.24%, Table 1). In retrospect, these elevations in FENa are speculated to be attributable to increased sodium excretion due to intensive i.v. fluid therapy begun immediately after admission, as opposed to progression to acute tubular necrosis [17].
Management of rhabdomyolysis includes correction of underlying causes, volume resuscitation, AKI prevention, and treatment of metabolic complications [3]. Aggressive fluid replacement with isotonic saline is first recommended to minimize the occurrence of renal failure. Mannitol may be protective owing to the associated diuresis that represses intratubular heme pigment deposition. Furthermore, alkalinization of the urine with administration of i.v. bicarbonate has been suggested to alleviate further renal injury by inhibiting myoglobin casts formation in the tubule. The administration of mannitol and/or sodium bicarbonate has been accepted as a standard therapy for the prevention of rhabdomyolysis-associated AKI; however, clinical evidence supporting the effectiveness of these medications is lacking [11]. Initiation of renal replacement therapy should be considered if exacerbation of metabolic acidosis (pH <7.1), resistant hyperkalemia (>6.5 mEq/L), volume overload, or prolonged anuria (≥12 h) has occurred despite optimal supportive therapy [11,18]. Conventional hemodialysis or continuous renal replacement therapy may be preferred in correcting fluid, electrolyte and acid–base abnormalities. Peritoneal dialysis is not an efficient renal replacement therapy for the removal of large solute loads in patients with rhabdomyolysis-associated AKI. The removal of myoglobin by plasmapheresis did not show any significant benefit [11,18].
At the onset of rhabdomyolysis, the risk factors for AKI and death include preexisting sepsis, dehydration, marked elevation of serum CK level, hyperkalemia, hyperphosphatemia, and hypoalbuminemia [19]. A study of 202 patients with rhabdomyolysis identified old age, reddish urine, trauma, and increased serum BUN, Cr, potassium, CK, and myoglobin levels as predictors of AKI. In particular, peak serum CK levels (>5000 U/L) and elevated serum myoglobin levels (>600 ng/mL) were identified as independent risk factors for AKI [20]. In the present report, hypovolemia, erythruria, and marked elevation of serum CK and myoglobin levels observed in our 2 patients were likely significant risk factors for AKI associated with rhabdomyolysis. The McMahon risk score, which is calculated based on clinical parameters at the time of admission, is a validated tool for predicting mortality or renal failure requiring renal replacement therapy in patients with rhabdomyolysis [21]. A McMahon score of 6 or higher had a higher sensitivity and specificity for predicting adverse clinical outcomes, including the need for renal replacement therapy, than did increased serum CK concentration (>5000 U/L) (86% vs 83% and 68% vs 55%, respectively) [21,22]. Both our patients had McMahon risk scores higher than 10 (11 and 12.5) at the time of admission (Table 1); therefore, their risk of death or need of dialysis was estimated to be 52% to 61.2% [21]. However, the patient in Case 1 showed improvements in rhabdomyolysis and azotemia with only antibiotic and fluid therapies, without implementing hemodialysis. On the contrary, the patient in Case 2 showed a rapid resolution of severe AKI-related complications, including acute pulmonary edema and metabolic acidosis, following 3 sessions of intermittent hemodialysis. Further studies on a larger number of patients will be required to identify the clinical predictors of disease severity, including the incidence of AKI, need for renal replacement therapy, and in-hospital mortality, in patients with rhabdomyolysis induced by
The first-line therapy for nontyphoidal
Conclusions
We described 2 patients with
Figures
References:
1.. Kaye KS, Kaye D, Salmonella infections (including typhoid fever).: Cecil Medicine, 2016; 1971-75, Philadelphia, PA, Saunders Elsevier
2.. Gabow PA, Kaehny WD, Kelleher SP, The spectrum of rhabdomyolysis: Medicine (Baltimore), 1982; 61; 141-52
3.. Cabral BMI, Edding SN, Portocarrero JP, Lerma EV, Rhabdomyolysis: Dis Mon, 2020; 66; 101015
4.. Allison RC, Bedsole DL, The other medical causes of rhabdomyolysis: Am J Med Sci, 2003; 326; 79-88
5.. Singh U, Scheld WM, Infectious etiologies of rhabdomyolysis: Three case reports and review.: Clin Infect Dis, 1996; 22; 642-49
6.. Rheingold OJ, Greenwald RA, Hayes PJ, Tedesco FJ, Myoglobinuria and renal failure associated with typhoid fever.: JAMA, 1977; 238; 341
7.. Zhu Z, Aghaie S, Bandarchuk A, Gandhi A, Rhabdomyolysis and acute kidney injury in Salmonella gastroenteritis: A case report.: Int J Case Rep Images, 2015; 6; 233-38
8.. Brncic N, Viskovic I, Sasso A, Salmonella infection-associated acute rhabdomyolysis. Some pathogenic considerations.: Arch Med Res, 2002; 33; 313-15
9.. Khan FY, Al-Ani A, Ali HA, Typhoid rhabdomyolysis with acute renal fail-ure and acute pancreatitis: A case report and review of the literature.: Int J Infect Dis., 2009; 13; e282-85
10.. Abdulla AJ, Moorhead JF, Sweny P: Nephrol Dial Transplant, 1993; 8; 672-73
11.. Bosch X, Poch E, Grau JM, Rhabdomyolysis and acute kidney injury: N Engl J Med, 2009; 361; 62-72
12.. , KDIGO clinical practice guideline for acute kidney injury: Kidney Int Supplements, 2012; 2; 1-138
13.. Neau D, Delmas Y, Merville P: Eur J Clin Microbiol Infect Dis, 2000; 19; 973-75
14.. Fleckenstein JL, Chason DP, Bonte FJ, Assessment of muscle viability with MR imaging and Tc-99m pyrophosphate scintigraphy: Radiology, 1995; 195; 205-10
15.. Long S, Garrett J, Bhargava P, Multimodality imaging findings in rhabdomyolysis and a brief review of differential diagnosis: Emerg Radiol, 2017; 24; 387-92
16.. Lee JW, Lee EY, Hong SY, Yoo ID, Lee SM, The clinical significance of technetium-99m methylene diphosphate bone scintigraphy findings in patients with rhabdomyolysis: Nucl Med Commun, 2017; 38; 820-25
17.. Perazella MA, Coca SG, Traditional urinary biomarkers in the assessment of hospital-acquired AKI: Clin J Am Soc Nephrol, 2012; 7; 167-74
18.. Petejova N, Martinek A, Acute kidney injury due to rhabdomyolysis and re-nal replacement therapy: A critical review.: Crit Care, 2014; 18; 224
19.. Ward MM, Factors predictive of acute renal failure in rhabdomyolysis: Arch Intern Med, 1988; 148; 1553-57
20.. Chen CY, Lin YR, Zhao LL, Clinical factors in predicting acute renal failure caused by rhabdomyolysis in the ED: Am J Emerg Med, 2013; 31; 1062-66
21.. McMahon GM, Zeng X, Waikar SS, A risk prediction score for kidney failure or mortality in rhabdomyolysis: JAMA Intern Med, 2013; 173; 1821-28
22.. Simpson JP, Taylor A, Sudhan N, Rhabdomyolysis and acute kidney injury: Creatine kinase as a prognostic marker and validation of the McMahon Score in a 10-year cohort: A retrospective observational evaluation: Eur J Anaesthesiol, 2016; 33; 906-12
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250