29 August 2022: Articles 
Metachronous Testicular Metastases from Merkel Cell Carcinoma (MCC): A Case Report and Literature Review
Rare disease
Alice Laffi1ABEF*, Gabriele CozziDOI: 10.12659/AJCR.936552
Am J Case Rep 2022; 23:e936552
Abstract
BACKGROUND: Merkel cell carcinoma (MCC) is a rare neuroendocrine neoplasm. The immunotherapy era has dramatically changed MCC prognosis, but unresponsive and progressive diseases after anti- programmed death-ligand 1 (PDL1) treatment still represent a challenge. MCC can metastasize in virtually every anatomical site, also during immunotherapy, and the reasons for primary resistance are debated. Testes are a rare metastatic site, accounting only 0.04% of autoptic detections in patients with more common malignancies. We report a case of a patient with metachronous and bilateral testes metastases after a previous radically treated MCC.
CASE REPORT: A 57-year-old man underwent radical surgery for left knee MCC. The oncologic follow-up was negative until 4 years later when the patient developed a right testis lesion. The patient underwent right orchiectomy for the suspicion of testicular primary malignancy, but the pathologist report revealed MCC metastasis. Subsequent radiologic assessment detected new bone metastases. The patient was treated with immunotherapy, experiencing a complete response. After 20 months of treatment, a further assessment revealed a new single left testis metastasis. A left orchiectomy was performed and immunotherapy was continued, maintaining a complete response.
CONCLUSIONS: There are few reports that MCC can be associated with uncommon metastases in testicular tissue. The present case suggests the testis represents another “sanctuary” site for the metastasizing process and host immune response. Considering the dramatic impact of immunotherapy in MCC prognosis, the study of these rare cases may aid the understanding of intrinsic resistance mechanisms to anti-PDL1, which affects a percentage of MCC patients.
Keywords: Carcinoma, Merkel Cell, Carcinoma, Neuroendocrine, Immunotherapy, Humans, Male, Middle Aged, Neoplasms, Germ Cell and Embryonal, Neoplasms, Second Primary, Prognosis, Skin Neoplasms, Testicular Neoplasms
Background
Merkel cell carcinoma (MCC) is a rare, high-grade neuroendocrine neoplasm, characterized by aggressive biologic behavior and a poor prognosis [1]. The immunotherapy era has dramatically changed the survival of patients with MCC, achieving the approval of anti-programmed death-ligand 1 (PDL1) as first line treatment for metastatic disease [2].
Despite the great results in prognosis, progressive diseases and those unresponsive to immunotherapy still represent an oncologic challenge whose causes have not yet been clarified by the scientific community. In these cases, MCC still presents a rapidly progressive behavior, and the ability to virtually metastasize at every anatomic site can lead to a quick decline in performance status, worsening of symptoms, and patient death. Several cases of uncommon metastatic sites have been reported in the literature, such as the tongue, heart, and parathyroid, suggesting MCC has a more unusual and aggressive behavior than other malignancies. Testes are in general a rare metastatic site, accounting for only 0.04% of autoptic detections in patients with more common malignancies [3], and only 11 cases of testicular MCC metastases are reported in the literature. We report the case of a male patient with metachronous and bilateral testes MCC. We conducted a review of the literature on the topic and analyzed the potential role of the host immune system in the metastasizing process.
Case Report
In June 2020, a 57-year-old man developed a right testicular mass. According to his medical history, in March 2016, the patient had been radically treated with surgery and adjuvant radiotherapy for a previous MCC of the left knee. He had no comorbidities and was not on any medications.
An ultrasound detected a 1.6×1.4-cm solid lesion of the right testis (Figure 1), and a fluorodeoxyglucose 18 F (18FDG)-positron emission tomography-computed tomography (PET/CT) scan confirmed a high uptake only in the right testicle.
Alpha-fetoprotein and beta-chorionic gonadotropin were in the reference range, while the lactate dehydrogenase level was 258 UI/L (reference range, 125–220). Placental-like alkaline phosphate was not dosed. In July 2020, the patient underwent a right orchiectomy, but the pathologic report revealed an MCC metastasis (with Ki-67 of 70% and immunohistochemistry positivity in synaptophysin, chromogranin A, and Merkel cell polyomavirus). The radiologic assessment after the orchiectomy revealed an 18FDG uptake in bone metastases (Figure 2), and a systemic treatment with an anti-PDL1 was started. After 3 months, a CT scan showed a progressive disease of new subcutaneous and large peritoneal lesions. Considering the good performance status, we decided to continue the treatment with immunotherapy, and after 6 weeks, CT and 18FDGPET/CT scanning showed a complete response (Figure 3), with undetectable distant metastases on both imaging results.
After 20 doses of the anti-PDL1 treatment (almost 10 months), in November 2021, the patient reported a progressive enlargement of the left testicle and the physical examination revealed a new left testicular mass. An ultrasound confirmed a solid lesion of the left testicle, and 18FDG-PET/CT showed an uptake only in the left testicle (SUVmax 12) (Figure 4). Therefore, in December 2021, the patient underwent a further left orchiectomy, and the pathologic report confirmed the diagnosis of Merkel cell polyomavirus-positive metastases.
After the discharge, the treatment with the same immunotherapy was continued. At the time of this report (5 months after the left orchiectomy), the patient was still alive with complete response shown on the radiologic assessment.
Discussion
Since the introduction of immunotherapy, the prognosis of patients with MCC has dramatically changed. According to the JAVELIN Merkel 200 study, patients treated with anti-PDL1 therapy experienced a great and durable response compared with those treated with platinum-based chemotherapy, which was the most used first-line regimen until the introduction of immunotherapy [2]. The 48-month overall survival and response rates were 31% and almost 40%, respectively, with an objective response rate of 75%, as recently presented at the American Society of Clinical Oncology (ASCO) 2021. According to the study by Weppler et al, presented as an abstract at the 2021 ASCO annual meeting I, MCC biology (which concerns mutational pattern and the growth rate), the host immune system, and the duration of anti-PDL1 treatment represent the main factors influencing the rate and durability of response to immunotherapy.
The study reported that 4% of relapses involved the central nervous system (CNS); interestingly, all CNS relapses seemed to occur in patients without previous brain involvement, suggesting immunotherapy was less effective in preventing metastases in this site. One of the most relevant reasons could be the brain-blood barrier (BBB), the system that controls brain homeostasis and, at the same time, limits the flow of extra-cranial factors [4]. When this system is disrupted by tumor progression, the BBB paradoxically seems to protect the metastases from systemic treatments, leading to a different response compared with all the other extracranial sites. Due to this phenomenon, the term “sanctuary” has been introduced to define the CNS, including all tissues protected by a blood-tissue barrier [5].
Less known and less common, the testicle represents the other tissue’s sanctuary, where the blood-testis barrier (BTB) is designed to preserve a man’s fertility [6]. In this site, Sertoli cells and immunoregulatory factors achieve a synergistic biochemical and immuno-protective action on the testicle environment. Therefore, the anatomic site and the BTB seem to represent the most likely reasons that a metastatic involvement of the testes is generally uncommon.
In this scenario, considering the incidence of MCC metastases of the genitourinary tract, metachronous, and subsequently (one occurring 10 months after the other) the testes, MCC metastases as in the present case is even a rarer occurrence. In Table 1 we reported the results of a review of the literature on this topic: only 11 cases of testicular metastases have been reported [7–15].
From comparing the present case with the others shown in Table 1, it is seen that a single testis relapse does not seem to be associated with systemic disease. The 4 cases developing a single testis MCC metastasis without other extra-testicular lesions showed a long survival (≥12 months) without further relapses after the radical surgery treatment. In our case, the patient developed bone metastases after 3 months of the first orchiectomy, suggesting that the testicular single relapse anticipated a systemic disease. After the second orchiectomy, during the immunotherapy, and until the time of the submission (5 months after), the patient did not develop other distant metastases, as in the case presented by Moscoloni et al [15]. The authors did not specify the time of follow-up, but the patient experienced a complete radiologic response after the orchiectomy and during immunotherapy. For our case, a further follow-up is needed to confirm the long-lasting complete radiologic response.
Compared with the other cases in Table 1, ours is the first case of metachronous and bilateral testicular MCC metastases in a patient receiving immunotherapy. Later than the other cases, the first testis MCC relapse occurred 52 months after the radical surgery of the primary malignancy.
Another 2 cases of metachronous and bilateral testes MCC metastases are reported in literature.
While they both described the testicular metastases occurrence as 3 months apart, our patient experienced the second testis relapse after 10 months of systemic treatment, suggesting that immunotherapy might have delayed a further progressive disease. According to this hypothesis, after the second orchiectomy, the patient experienced a complete radiologic response until the time of the submission (5 months after radical surgery), continuing to be treated with immunotherapy.
Two observations about the role of BTB and MCC testis relapses can be made.
First, BTB may contribute to the creation of another “sanctuary” site for uncommon metastasizing, protecting this environment by the host innate immune system and leading to the metastases with a different behavior than that of other extra-testicular sites. This hypothesis may justify the occurrence of the first testis metastasis in our case.
Second, BTB may also represent the reason for the lack of response to immunotherapy in testicular tissue by preventing the effect of the systemic treatment. This hypothesis may justify the occurrence of the second testis relapse in our case and the maintaining of the radiologic complete response on all the other extra-testicular sites. Several authors have already speculated on a similar “sanctuary” effect: underling the role of the BBB on the CNS in terms of systemic treatment resistance, they reported cases of patients with radiologic complete response and long survival after the radical treatment of single brain relapses from other malignancies [16,17].
Although a longer follow-up is needed for our case, BTB can be assumed to have for the testicular environment the same role of the BBB for brain tissue, and our patient may experience a long-lasting complete response.
Conclusions
The role of BTB in the immunotherapy response has not been prospectively analyzed. Considering the dramatic impact that immunotherapy produced on MCC prognosis, a percentage of patients still experience a primary resistance to anti-PDL1, whose mechanisms mostly remain unknown. The analysis of the present case suggests the testis may represent another “sanctuary” site for the metastasizing process and host immune response, where the BTB paradoxically produces relapse protection by the innate immune system, and the reason for the lack of response to immunotherapy in testicular tissue.
Through the study of these rare cases, it could be possible to better understand the intrinsic resistance of some malignancies to immunotherapy and to produce hypotheses to overcome these mechanisms.
Figures
References:
1.. Albores-Saavedra J, Batich K, Chable-Montero F, Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population based study: J Cutan Pathol, 2010; 37(1); 20-27
2.. D’Angelo SP, Lebbe C, Mortier L, First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): Primary and biomarker analyses of a phase II study: J Immunother Cancer, 2021; 9(7); e002646
3.. Ulbright TM, Young RH, Metastatic carcinoma to the testis: A clinicopathologic analysis of 26 nonincidental cases with emphasis on deceptive features: Am J Surg Pathol, 2008; 32(11); 1683-93
4.. Arvanitis CD, Ferraro GB, Jain RK, The blood-brain barrier and blood-tumour barrier in brain tumours and metastases: Nat Rev Cancer, 2020; 20(1); 26-41
5.. Palmieri D, Chambers AF, Felding-Habermann B, The biology of metastasis to a sanctuary site: Clin Cancer Res, 2007; 13(6); 1656-62
6.. Stanton PG, Regulation of the blood-testis barrier: Semin Cell Dev Biol, 2016; 59; 166-73
7.. Ro JY, Ayala AG, Tetu B, Merkel cell carcinoma metastatic to the testis: Am J Clin Pathol, 1990; 94(4); 384-89
8.. Rufini V, Perotti G, Brunetti M, Crescenzi A, Unsuspected testicular metastases from Merkel cell carcinoma: A case report with therapeutic implications: Am J Clin Oncol, 2004; 27(6); 636-37
9.. Gleason JM, Kohler TS, Monga M, Merkel cell carcinoma metastatic to testis: Urology, 2006; 67(2); 423.e13-e14
10.. Schwindl B, Meissner A, Giedl J, Klotz T, Merkel cell carcinoma – a rarity in the urogenital tract: Onkologie, 2006; 29(7); 326-28
11.. Tummala MK, Hausner PF, McGuire WP, CASE 1. Testis: A sanctuary site in Merkel cell carcinoma: J Clin Oncol, 2006; 24(6); 1008-9
12.. Whitman EJ, Brassell SA, Rosner IL, Moncur JT, Merkel cell carcinoma as a solitary metastasis to the testis: J Clin Oncol, 2007; 25(24); 3785-86
13.. Mweempwa A, Tan A, Dray M, Recurrent Merkel cell carcinoma of the testis with unknown primary site: A case report: J Med Case Rep, 2016; 10(1); 314
14.. Gigliano D, Lobo J, Lopes P, Merkel cell carcinoma metastatic to the testis: Report of a rare diagnosis and review of the literature: Autops Case Rep, 2021; 11; e2020198
15.. Lara Moscoloni LF, Miguel LE, Maximiliano S, Testicular metastasis of Merkel’s cell carcinoma: Urol Androl Open J, 2020; 4(3); 40-42
16.. Onesti CE, Iacono D, Angelini S, Unexpected long survival of brain oligometastatic non-small cell lung cancer (NSCLC) treated with multimodal treatment: A single-center experience and review of the literature: Transl Lung Cancer Res, 2016; 5(6); 712-19
17.. Sakurai H, Kurishima K, Homma S, Isolated solitary brain metastasis as a relapse of small cell lung cancer: Oncol Lett, 2013; 6(4); 1108-10
Figures
Tables
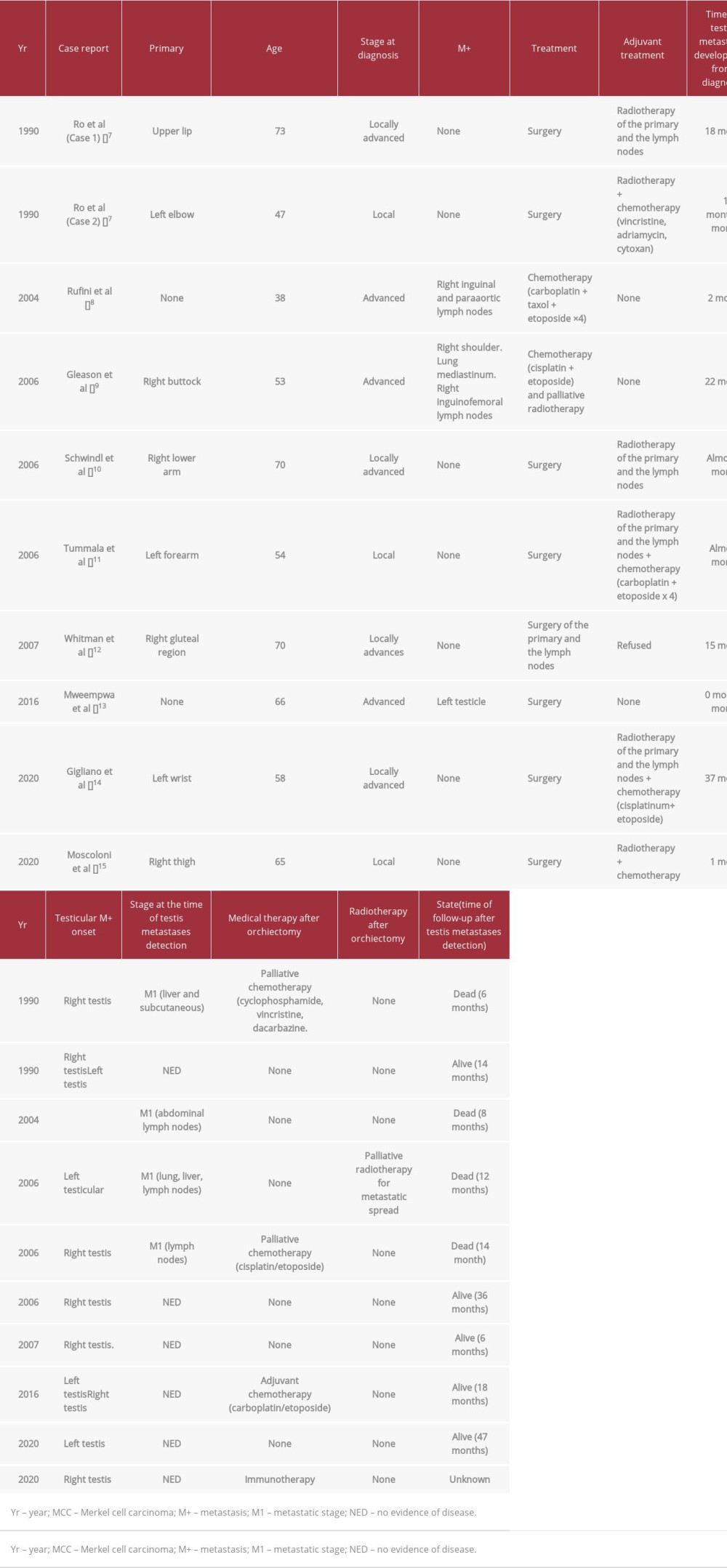 Table 1.. Cases of testis metastases of Merkel cell carcinoma reported in literature. Clinical, pathological, and therapeutic features of the MCC cases are reported. In last column, we reported (when available) the survival of the patients after the testis metastasis detection.
Table 1.. Cases of testis metastases of Merkel cell carcinoma reported in literature. Clinical, pathological, and therapeutic features of the MCC cases are reported. In last column, we reported (when available) the survival of the patients after the testis metastasis detection. Table 1.. Cases of testis metastases of Merkel cell carcinoma reported in literature. Clinical, pathological, and therapeutic features of the MCC cases are reported. In last column, we reported (when available) the survival of the patients after the testis metastasis detection.
Table 1.. Cases of testis metastases of Merkel cell carcinoma reported in literature. Clinical, pathological, and therapeutic features of the MCC cases are reported. In last column, we reported (when available) the survival of the patients after the testis metastasis detection. In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








