26 September 2022: Articles 
Alleviation of Post-COVID-19 Cognitive Deficits by Treatment with EGb 761: A Case Series
Unusual or unexpected effect of treatment
Udo A. Zifko1E*, Muhammad Yacob1E, Benedikt J. Braun1BE, Gunnar P.H. Dietz2EFDOI: 10.12659/AJCR.937094
Am J Case Rep 2022; 23:e937094
Abstract
BACKGROUND: Cognitive symptoms persisting longer than 3 months after infection, such as memory loss, or difficulties concentrating, have been reported in up to one-third of patients after COVID-19. Evidence-based therapeutic interventions to treat post-COVID-19 symptoms (also called “Long-COVID symptoms”) have not yet been established, and the treating physicians must rely on conjecture to help patients. Based on its mechanism of action and its efficacy in treating cognitive impairment, as well as its good tolerability, the Ginkgo biloba special extract EGb 761 has been suggested as a remedy to alleviate cognitive post-COVID-19 symptoms. In many studies, EGb 761 has been demonstrated to protect endothelial cells, to have potent anti-inflammatory effects, and to enhance neuroplasticity.
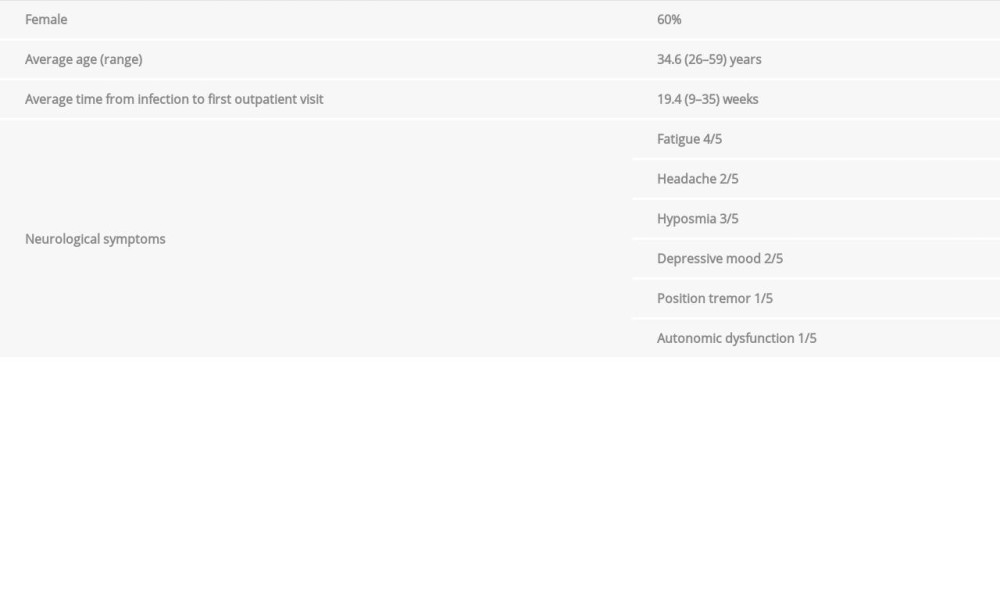
CASE REPORT: Here, we report for the first time the application of EGb 761 in the therapy of post-COVID-19-related cognitive deficits. Three women and 2 men, aged 26 to 59 years (average age 34.6 years), presented with concentration and attention deficits, cognitive deficiencies, and/or fatigue 9-35 weeks after infection. A daily dose of 2×80 mg of EGb 761 did not cause any detectable adverse effects, and it substantially improved or completely restored cognitive deficits and, when initially present, also other symptoms, such as fatigue and hyposmia, within an observation period of up to 6 months.
CONCLUSIONS: Our observations support the hypothesis that EGb 761 might be a low-risk treatment option for post-COVID-19 patients with cognitive symptoms. Moreover, we derive recommendations for randomized controlled clinical trials to confirm efficacy in that indication.
Keywords: SARS-CoV-2, cognitive dysfunction, post-acute COVID-19 syndrome, Neurocognitive Disorders, Adult, Anti-Inflammatory Agents, COVID-19, Cognition, endothelial cells, Fatigue, Female, Ginkgo biloba, Humans, Male, Plant Extracts, post-acute COVID-19 syndrome
Background
The global pandemic with the SARS-CoV-2 virus started in December 2019. The acute disease is often asymptomatic or benign, while in certain patients, especially those with risk factors such as advanced age, a multitude of severe symptoms can develop [1], which includes a plethora of neurological symptoms [2]. Results by our own research group showed that 83% of patients with acute COVID-19 displayed nervous system symptoms [3]. This was recorded in both the outpatient setting and the inpatient setting at approximately equal incidences. The majority (63.4%) of neurologic symptoms occurred on the first or second day of illness and were characterized by the simultaneous occurrence of multiple neurologic symptoms in nearly three-quarters of all patients. Those symptoms can persist long after infection, and can frequently occur even in patients with an initially mild disease course [4]. Depending on patient selection and methodology of the assessment, in single studies, up to three-quarters of patients had post-COVID-19 symptoms such as long-term fatigue or exhaustion, up to one-third had memory dysfunction, and up to one-quarter had cognitive deficits [5].
The reasons for frequent neurological or psychiatric post-COVID-19 symptoms are not clear and are certainly complex and depend on many factors, including neuroinvasiveness and neurotropism of SARS-CoV-2 [2]. Some evidence suggests that the virus might cross the blood-brain barrier by damaging the endothelial cell layer [2]. Moreover, it may induce neurovascular complications by inflammatory processes and endothelial dysfunction [6]. Evidence also suggests that COVID-19 disease mechanisms involve endothelial damage; receptors with an affinity for the viral spike protein are expressed on endothelial cells, and damage to endothelial cells could explain much of the pathology of multiple organ dysfunctions observed in COVID-19 [7]. Not only in acute COVID-19, but also in post-COVID-19, endothelial damage appears to play a role. For instance, increased endothelial progenitor production, which is an indicator of vascular damage, is present in post-COVID-19 patients [8]. Besides endothelial dys-function, they also display altered endothelial biomarkers [9].
The
Although 59 trials investigating treatment of post-COVID symptoms have been recently registered [30], to date, no therapeutic regimens have been established for such patients. However, for the above reasons, the use of Ginkgo extract in COVID-19 infections has been suggested [24].
Here, we describe the results from the treatment of 5 patients with
Case Reports
PATIENTS AND TREATMENT:
We established a post-COVID-19 outpatient clinic with exclusive care of neurological deficits in July 2021. Based on our experience, cognitive deficits represented the main concern for many patients, especially in terms of professional reintegration but also in terms of sufficient management of daily activities. The COVID-19 disease course was scored to be either mild, moderate, critically, or severely affected according the COVID-19 infection severity scale [31]. SARS-CoV-2 infection status was initially determined using commercial antibody tests, confirmed by PCR analysis. None of the patients required hospitalization for acute COVID-19 treatment.
From 5 outpatients who had SARS-CoV-2 infection between December 2020 and July 2021, with later concerns about cognitive dysfunction, demographic and anamnestic data were assessed, as well as the neuropsychological performance using the Montreal Cognitive Assessment (MoCA) [32] and neurological status (Table 1). Specific neurological examinations, MRI brain imaging, and EEG were performed according to symptomatology and clinical findings.
The extent of cognitive deficits was quantified on the day of the initial assessment using the Clinical Global Impression Severity (CGIS) scale and the recording of change at follow-up using the Clinical Global Improvement or Change (CGIC) scale [33]. The CGIS is a numerical rating scale of severity of illness, ranging from 1 (normal, not at all ill) to 7 (among the most extremely ill patients). The initial scales detected among the 5 patients were 4 (moderately ill), 5 (markedly ill), and 6 (severely ill). CGIC is a numerical rating scale of therapeutic drug effects, ranging from 1 to 16, with 1 meaning complete or nearly complete remission of all symptoms with no side effects; 5 meaning decided improvement with partial remission of symptoms and no side effects; and 16 meaning unchanged or worse, with adverse effects outweighing the benefits.
Patients were given 2 x 80 mg EGb 761® daily. EGb 761® is a dry extract from
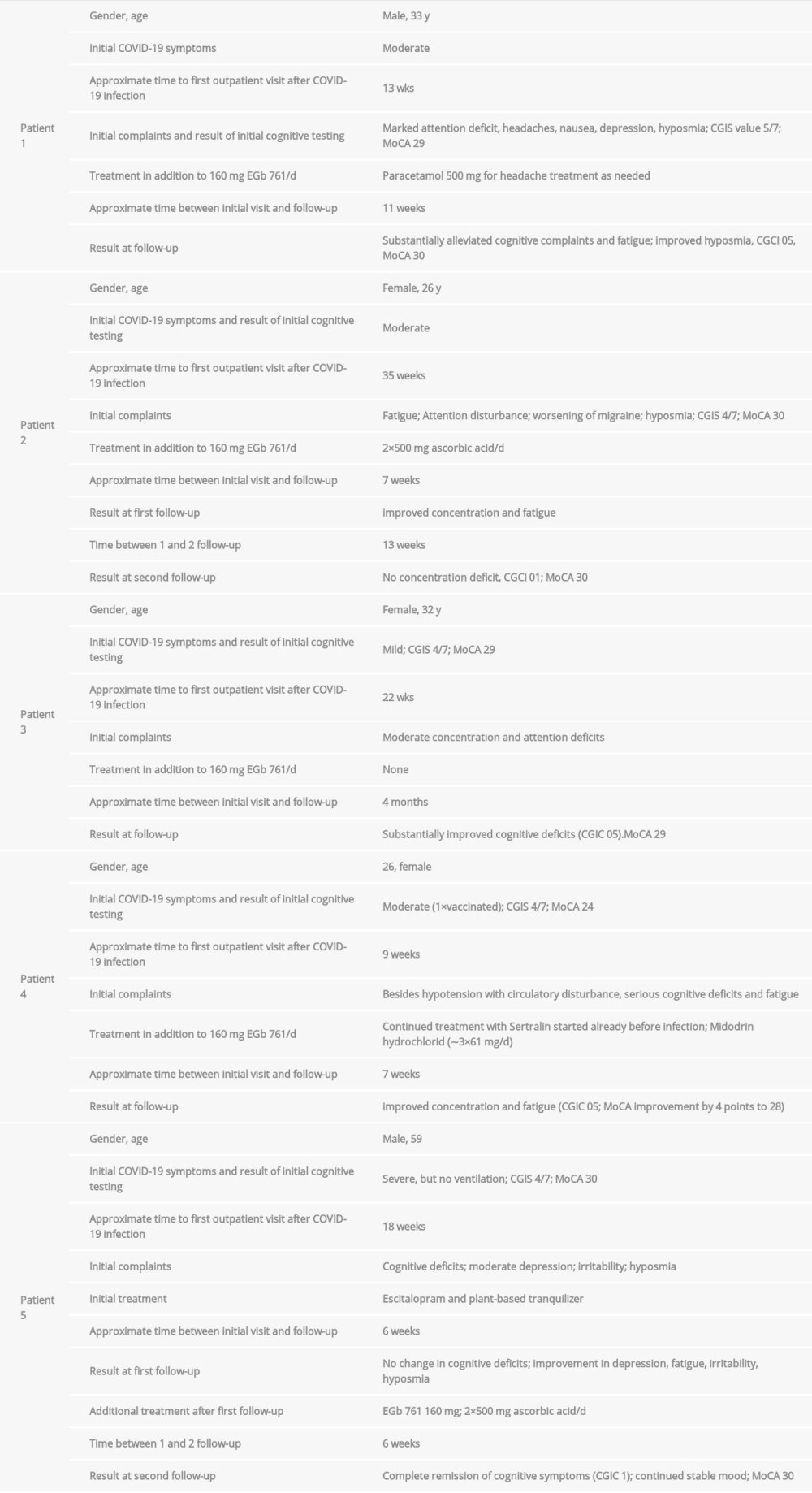
CASE 1:
The 33-year-old male skilled worker was infected with SARSCoV-2 at the end of March 2021, with a moderate disease course. He had not been vaccinated at the time of infection. During acute COVID infection, he mainly had headache, fever up to 38.5°C, sore throat, hyposmia, joint and body aches, and dry cough over a period of weeks. Hospitalization was not required. In addition to shortness of breath for about 8 weeks after the first positive PCR test, he visited the outpatient clinic for the first time on July 2, 2021 because of attention deficit, CGIS value 5/7, 3–4×weekly headache symptoms with nausea, fatigue, and depressed mood. The patient had a history of insulin-dependent diabetes mellitus and transient focal epilepsy in 2017, which had been treated with levetiracetam over 1 year. Cognitive symptoms limited his job activities but did not interfere with private activities.
The neurological status showed a diminished sense of smell, with otherwise unremarkable findings. MRI of the brain was unremarkable, with chronic sinusitis as a secondary finding. The EEG showed a flat curve pattern without abnormalities. The MoCA score was 29 points. The patient could only name 9 items beginning with the letter F.
In addition to 500 mg paracetamol for headache therapy as needed, the patient received 2×80 mg EGb 761® per day, starting on July 3, 2021. At the September 16, 2021 follow-up, the MoCA had improved to the maximum achievable score of 30. A substantial improvement in cognitive concerns (CGIC 05) was reported, in addition to a decreased perception of fatigue and an improvement in olfaction. No adverse events were reported.
CASE 2:
A 26-year-old woman, doctor of laws, experienced a moderate course of COVID-19 in December 2020 including fever of 38°C for 3–4 days, body aches, circulatory problems, and generalized weakness for 14 days. She had not been vaccinated at that time. Symptomatic treatment with antipyretic drugs and pain killers was performed at home. However, she had persistent fatigue symptoms and attention disturbance (CGIS 4/7), as well as transient worsening of the previously known migraine symptoms between March and June 2021. She mainly reported difficulties due to attention deficits in her daily job activities as a lawyer. On August 20, 2021, the patient first presented herself at the outpatient clinic. The neurological status also showed hyposmia. Otherwise, no objective abnormalities were detected. The MoCA score was 30.
After the administration of 2×80 mg EGb 761® in combination with 2×500 mg/d ascorbic acid starting on August 21, 2021, there was a significant improvement in concentration and a decrease in fatigue as early as October 9, 2021. The MoCA score remained unaltered at its maximum value of 30. According to a telephone follow-up after 3 months (January 8, 2022), concentration deficits had completely regressed (CGIC 01), and the medication was well tolerated.
CASE 3:
A 32-year-old woman trained as Bachelor for Arabic linguistics had mild COVID-19 in February 2021, including tension headache, tiredness, dull muscle pain, and sweating at night within the first 2 days, which was treated at home. She had not received a vaccination against SARS-CoV-2. On clinical-neurological examination on July 21, 2021, she presented with pre-existing left Horner’s syndrome and birth-related brachial plexus weakness. Concentration and attention deficits were substantial (CGIS 4/7) in family activities only, as she did not work during the time of COVID infection.
An MRI of the brain was unremarkable and the MoCA test result was 29 points. The repetition of 5 words after an interval of 1 min resulted in 1 missing word. The patient started on July 22, 2021 with 2×80 mg of EGb 761® daily. At the November 2021 follow-up, a marked improvement in cognitive deficits was documented (CGIC 05). The MoCA score increased to a value of 30. No adverse events were reported.
CASE 4:
A 26-year-old woman, master of journalism and working as freelance journalist, with 1 mRNA SARS-CoV-2 vaccination in June 2021, was infected with SARS-CoV-2 in July 2021 (prior to a planned second immunization) with a moderately severe disease course. She had hoarseness, painful swallowing, some shortness of breath and cough, and intense tiredness. She did not receive a booster vaccination. On September 10, 2021, she presented to our outpatient clinic. In addition to arterial hypotension with circulatory disturbances, position tremor, autonomic dysfunction, the patient suffered in particular from cognitive deficits with impaired concentration and attention, CGIS 4/7, and rapid fatigability. Before developing COVID-19, the patient was treated for depression with sertraline 50 mg 1-0-0, to which she had responded well, and treatment was continued throughout the observation period.
The clinical-neurological status was unremarkable. However, neuropsychological testing with the MoCA resulted in 24 points, which is below the suggested cut-off [32]. Clock-drawing test resulted in 3 of 5 possible points and only 2 of 5 words were recalled correctly after a period of 5 min. The patient was not able to do her work duties, and also asked for support in performing daily activities at home, such as shopping and cooking.
The patient received symptomatic therapy for orthostatic dys-regulation (3×7 drops Midodrin hydrochlorid/d≈183 mg/d) in addition to 2×80 mg/d EGb 761®.
At the follow-up examination on November 29, 2021, the MoCA score had improved to 28 points, and there was a significant improvement in the concentration disorder and also a reduction in fatigability, with no adverse effects as assessed by the CGIC rating (score of 5).
CASE 5:
A 59-year-old man working as commercial artist had a severe course of COVID-19 in March 2021, with fever above 38°C over a 2-week period and marked shortness of breath with generalized muscle weakness over several weeks. No ventilation was required, and the patient was treated at home. He had not been vaccinated against SARS-CoV-2. Post-COVID-19 symptoms persisted until initial assessment on July 14, 2021. At that time, he had moderate depression, fatigue, a low threshold irritability, and cognitive deficits interfering with job activities, and CGIS was 4/7. Neurological status showed hyposmia with otherwise unremarkable findings. The MoCA score was 30 and the DemTect score was 18.
At initial assessment, a purely antidepressant therapy with 20 mg Escitalopram/day as well as an herbal preparation in the evening to promote sleep – 1 capsule of “Relaxum”/d, containing extracts of lemon balm (90 mg), hops (28 mg), valerian root (100 mg), passionflower (100 mg) – was administered. At the first follow-up after 6 weeks, significant improvement of the depressive episode, fatigue, irritability, and olfactory disturbance was observed. However, the cognitive and attention deficits remained unchanged. Vitamin C (2×500 mg/d) and 2×80 mg/d EGb 761® were thus prescribed in addition with continued antidepressant therapy.
At an additional check-up 6 weeks later, he had a continued stable mood, almost complete improvement of cognitive deficits without adverse effects, and CGIC 01 was observed.
Discussion
Under EGb 761® treatment, among the 5 patients described here (data summarized in Table 2), 3 experienced a partial remission of their post-COVID-19 cognitive impairment (CGIC 5), while 2 showed a nearly complete remission of all cognitive symptoms (CGIC 1). None of the patients had any adverse effects of EGb 761® treatment. Moreover, neuropsychiatric symptoms and hyposmia also improved in several instances.
The present data suggest that symptomatic treatment of cognitive performance deficits in the context of post-COVID-19 with EGb 761® may be of substantial therapeutic help. This is plausible based on the known mechanisms discussed above.
Our patients reported concentration and attention deficits during everyday activities. During treatment, the MoCA test result increased by 4 points to a score of 28 in patient 4. In all other cases, the test score was close to or at the maximum achievable value of 30. Although clear subjective cognitive concerns were present before treatment, this deficit was in most cases not detectable using the MoCA or DemTect. This experience suggests that those tests are not sufficiently sensitive to be used as a screening tool for post-COVID-19-related cognitive disturbances, unless the symptoms are very severe.
To that end, a recent publication provided evidence for cognitive tests more amenable to detect post-COVID-19 symptoms [34], in which 401 patients who were infected with SARSCoV-2 aged 51 to 81 years showed several findings related to the cognitive changes described in this study. It was shown that at an average time of 141 days between the first brain scans and the second examination, after SARS-CoV-2 infection, significant effects were evident compared with subjects without infection. These included a greater reduction in gray matter in the orbitofrontal cortex and parahippocampal area compared with the control group. Furthermore, greater changes in the primary olfactory center and an overall greater reduction in brain size were also observed. The detailed neuropsychological examinations also showed a loss of cognitive abilities. That result could also be reproduced a period when only COVID-19 individuals who were not hospitalized were included. In the neuropsychological tests, the “Trail Making Test” proved to be particularly helpful. Thus, while memory tests were not sufficiently sensitive, attention and concentration deficits could be objectified. That report encompassed data from when the viral alpha variant was prevalent. To what extent this correlates with the now-current omicron variant remains to be determined. In the context of the cases presented here, patients with both the alpha variant and the delta variant have been described.
Patient 1, who also had hyposmia, regained his sense of smell during treatment. That observation is consistent with previous studies in which Ginkgo extract alleviated loss of olfaction due to viral infection [35,36]. In contrast, in healthy young adults without olfactory impairment, Ginkgo extract did not improve the sense of smell [37]. Possibly the already mentioned well-documented circulation-enhancing, antioxidant effect and the effect on brain adaptability (neuroplasticity) of Ginkgo extract, including in the olfactory brain [38] makes such an effect plausible. Furthermore, preclinical studies suggest that Ginkgo leaf extract may promote regeneration of the damaged olfactory sense [18,39,40].
Patient 5 showed an improvement of the depressive mood and impulse disturbance under general symptomatic therapy measures, but no improvement of the attention deficit. That improvement only occurred when treatment was supplemented with 160 mg EGb 761®.
Whereas most clinical trials demonstrating efficacy and safety of EGb 761® have been performed with older patients, here, successful treatment was shown with patients as young as 26 years. While it would be premature to assume that EGb 761® would be effective as a neuroenhancer in young, healthy people, it may be beneficial in the challenged brain, regardless of age.
In recent trials, 240 mg/d of EGb 761® has usually been used [41]. Whether an even stronger effect of the medication can be observed when higher doses than the 160 mg/d used in those 5 post-COVID-19 cases remains to be seen.
A big concern is that many post-COVID-19 patients, as in our study, bear their symptoms for many months before they make an appointment with a physician. Treatment might be more effective if it was started at the start of the disease rather than when cognitive and psychiatric symptoms have been established for months.
Our results are consistent with theoretical considerations suggesting that the use of EGb 761® is a promising therapeutic intervention for cognitive deficits in patients with post-COVID-19 conditions.
Conclusions
In a consecutive series of 5 cases, we observed clear and rapid improvement or complete regression of post-COVID-19 cognitive deficits during administration of EGb 761® (Figure 1). In many patients, at least some aspects of post-COVID-19-related cognitive impairment subside over time [42]. Thus, from a case series it is not clear which aspects of the recovery were due to the pharmacologic effects of EGb 761®. On the other hand, the fact that the cognitive deficits had been persisting for quite some time and were alleviated soon after EGb 761® was given suggests that a controlled clinical trial testing EGb 761® in the treatment of post-COVID-19 symptoms is warranted.
Furthermore, this case series shows that the MoCA only detects cases of clinically severe cognitive impairment in post-COVID-19 patients. Thus, for clinical assessment of cognitive post-COVID-19 symptoms, more sensitive neuropsychological tests should be used, which also address fatigue.
To treat patients with post-COVID-19-associated cognitive deficits, evidence-based therapeutic options have not yet been established. In light of the good tolerability and low interaction potential with other drugs, the use of EGb 761® at a daily dose of at least 160 mg is a therapeutic option to be considered.
References:
1.. Gallo Marin B, Aghagoli G, Lavine K, Predictors of COVID-19 severity: A literature review: Rev Med Virol, 2021; 31(1); 1-10
2.. Bauer L, Laksono BM, de Vrij FMS, Kushner SA, The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2: Trends Neurosci, 2022; 45; 358-68
3.. Zifko U, Schmiedlechner T, Saelens J, COVID-19: Involvement of the nervous system: Identifying neurological predictors defining the course of the disease. J Neurol Sci, 2021; 425; 117438
4.. Mehandru S, Merad M, Pathological sequelae of long-haul COVID: Nat Immunol, 2022; 23(2); 194-202
5.. Nasserie T, Hittle M, Goodman SN, Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review: JAMA Netw Open, 2021; 4(5); e2111417
6.. Reddy ST, Garg T, Shah C, Cerebrovascular disease in patients with COVID-19: A review of the literature and case series: Case Rep Neurol, 2020; 12(2); 199-209
7.. Sardu C, Gambardella J, Morelli MB, Hypertension, thrombosis, kidney failure, and diabetes: Is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence: J Clin Med, 2020; 9(5); 1417
8.. Poyatos P, Luque N, Eizaguirre S, Post-COVID-19 patients show an increased endothelial progenitor cell production: Transl Res, 2022; 243; 14-20
9.. Haffke M, Freitag H, Rudolf G, Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS): J Transl Med, 2022; 20(1); 138
10.. Song J, Liu D, Feng L: Evid Based Complement Alternat Med, 2013; 2013; 846126
11.. Wang Y, Pei DS, Ji HX, Xing SH, Protective effect of a standardized Ginkgo extract (ginaton) on renal ischemia/reperfusion injury via suppressing the activation of JNK signal pathway: Phytomedicine, 2008; 15(11); 923-31
12.. Naidu MU, Shifow AA, Kumar KV, Ratnakar KS: Phytomedicine, 2000; 7(3); 191-97
13.. Schütt F, Aretz S, Auffahrt GU, Kopitz J, [Role of energy metabolism in retinal pigment epithelium]: Ophthalmologe, 2013; 110(4); 346-52 [in German]
14.. Behar-Cohen FF, Heydolph S, Faure V, Peroxynitrite cytotoxicity on bovine retinal pigmented epithelial cells in culture: Biochem Biophys Res Commun, 1996; 226(3); 842-49
15.. Liu KX, He W, Rinne T: Am J Chin Med, 2007; 35(5); 805-19
16.. Chen JW, Chen YH, Lin FY: Arterioscler Thromb Vasc Biol, 2003; 23(9); 1559-66
17.. Wu YZ, Li SQ, Zu XG: PhytotherRes, 2008; 22(6); 734-39
18.. Lee CH, Mo JH, Shim SH: AmJRhinol, 2008; 22(3); 292-96
19.. Mansour SM, Bahgat AK, El-Khatib AS, Khayyal MT: Phytomedicine, 2011; 18(8–9); 641-47
20.. Zhang XH, Zhang M, Wu JX: Br Poult Sci, 2020; 61(2); 180-87
21.. Qiao P, Yan H, Wang J: Neurochem Res, 2020; 45(10); 2398-408
22.. Chiu YL, Tsai WC, Wu CH: Am J Chin Med, 2020; 48(2); 357-72
23.. Diamond BJ, Shiflett SC, Feiwel N: Arch Phys Med Rehabil, 2000; 81(5); 668-78
24.. Ibrahim MA, Ramadan HH, Mohammed RN: J Basic Clin Physiol Pharmacol, 2021; 32(3); 131-43
25.. Tchantchou F, Lacor PN, Cao Z, Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons: J Alzheimers Dis, 2009; 18(4); 787-98
26.. Tchantchou F, Xu Y, Wu Y: Faseb J, 2007; 21(10); 2400-8
27.. Fehske CJ, Leuner K, Müller WE: Pharmacol Res, 2009; 60(1); 68-73
28.. Eckert A, Keil U, Kressmann S: Pharmacopsychiatry, 2003; 36(Suppl. 1); S15-S23
29.. Kellermann AJ, Kloft C: Pharmacotherapy, 2011; 31(5); 490-502
30.. Ceban F, Leber A, Jawad MY, Registered clinical trials investigating treatment of long COVID: A scoping review and recommendations for research: Infect Dis (Lond), 2022; 54(7); 467-77
31.. , Hinweise zu Erkennung Diagnostik und Therapie von Patienten mit COVID-19 (). 2020https://edoc.rki.de/bitstream/handle/176904/6511/STAKOB-Therapie_2020-03-13-14h_Hyperlinks-14Maerz13h.pdf?sequence=1
32.. Nasreddine ZS, Phillips NA, Bedirian V, The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment: J Am Geriatr Soc, 2005; 53(4); 695-99
33.. Guy W, CGI Clinical Global Impressions: ECDEU assessment manual for psychopharmacology, 1976; 217-22, Rockville, Md, U.S. Dept. of Health, Education, and Welfare
34.. Douaud G, Lee S, Alfaro-Almagro F, SARS-CoV-2 is associated with changes in brain structure in UK Biobank: Nature, 2022; 604(7907); 697-707
35.. Seo BS, Lee HJ, Mo JH: Arch OtolaryngolHead Neck Surg, 2009; 135(10); 1000-4
36.. Guo YC, Yao LY, Wei YX: Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 2017; 31(20); 1585-88 [in Chinese]
37.. Mattes RD, Pawlik MK: Hum Psychopharmacol, 2004; 19(2); 81-90
38.. Droy-Lefaix MT: Age (Omaha), 1997; 20(3); 141-49
39.. Lee GS, Cho JH, Park CS: Auris Nasus Larynx, 2009; 36(3); 287-91
40.. Didier A, Jourdan F: Cell Mol Biol (Noisy-le-grand), 2002; 48(6); 717-23
41.. Gauthier S, Schlaefke S: Clinical Interventions in Aging, 2014; 9; 2065-77
42.. Ferrucci R, Dini M, Rosci C, One-year cognitive follow-up of COVID-19 hospitalized patients: Eur J Neurol, 2022; 29(7); 2006-14
In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250