21 December 2022: Articles 
Masson Tumor of the Central Nervous System: A Case Report and Review of Literature
Challenging differential diagnosis, Diagnostic / therapeutic accidents, Rare disease
Fatemeh MohammadyariDOI: 10.12659/AJCR.937597
Am J Case Rep 2022; 23:e937597
Abstract
BACKGROUND: Masson’s tumor, also known as intravascular papillary endothelial hyperplasia (IPEH), is an unusual endothelial proliferation that leads to improper thrombus development due to faulty endothelial structure. Although IPEH is rare in the central nervous system, it can arise at any location in the brain. Headaches, seizures, and focal neurological symptoms ae the most common presenting symptoms. It is more common in females and it can occur at any age.
CASE REPORT: Herein, we present a 65-year-old female patient with a progressively enlarging right temporal lobe mass that was initially considered metastatic ovarian carcinoma. She underwent a right temporal craniotomy and the lesion was totally resected. Contrary to expectations, the pathology report was an IPEH.
CONCLUSIONS: In this paper, we conducted a literature review of previously reported cerebral IPEH cases, with a focus on their clinical and radiological presentations, management, and especially their association with previous radiotherapy. The important point is that one-third of the cases had a history of radiation therapy to the head, and most of them had stereotactic radiosurgery (SRS) on the location of the brain from which IPEH subsequently developed. The major question for which we are looking for an answer is its relationship with previous radiotherapies. We wanted to know how many of these cases were associated with radiotherapy in the same area, the time interval from radiotherapy to the onset of IPEH or symptoms, the dose of the previous radiotherapy, and, overall, if there is any cause-effect relationship between IPEH and radiotherapy.
Keywords: Brain Neoplasms, Cerebellar Neoplasms, Female, Humans, Aged, Central Nervous System, Endothelium, Vascular, Vascular Neoplasms, Brain, Central Nervous System Neoplasms
Background
Intravascular papillary endothelial hyperplasia, commonly known as Masson’s tumor, is a reactive, slow-growing, nonneoplastic exuberant recanalization with papillary formations with hyaline or fibrous stalks, and anastomosing vascular channels. There is no necrosis, atypia, or increased mitotic figures, and it is frequently associated with organized thrombus in the presence of angiogenic factors, accounting for around 2% of all vascular tumors [1–4]. Pierre Mason first reported IPEH as a vegetant intravascular hemangioendothelioma in an ulcerous hemorrhoidal vein in a patient in 1923 [5]. IPEH, which was initially described by Weheb et al in 1986 [6], has been characterized as developing in a variety of places throughout the body, including in cutaneous and subcutaneous tissue; however, cerebral involvement is rare and manifests with mass effects or neurological impairments based on its anatomical location [7–10]. Although this entity is benign, IPEH is usually detected after surgical resections. There are no distinct radiological features that can differentiate it from other benign or malignant brain tumors or vascular malignancies like angiosarcoma [11,12]. It usually presents as a mass, isointense to hypointense in T1 and hyperintense in T2, with different degrees of enhancement after gadolinium injection. There may be significant peri-lesional edema and mass effect; therefore, despite its deceiving nature, intracranial IPEH can be fatal as a result of mass effect and brain herniation. Complete resection, if feasible, is the treatment of choice, but adjuvant treatment (eg, with anti-angiogenic agents) is needed if complete surgical resection is not possible [10,13–15].
Regardless of many case reports, its relationship with the previous radiotherapy has remained unclear. Therefore, we presented this case and conducted this review of the literature to determine if there is any strong epidemiological link between this disease entity and previous radiotherapies.
Case Report
A 65-year-old woman who was diagnosed with stage IV ovarian carcinoma in 2013 presented with progressive headaches that she had experienced before. Nine years previously, she had undergone chemotherapy and debulking surgery. Two years later, her brain MRI showed a left parietal brain solitary metastasis that required resection and stereotactic radiosurgery (SRS) at the surgical site. Her follow-up MRI in 2016 showed 3 new lesions in the right temporal, right cerebellar peduncle, and right cerebellar hemisphere, for which she underwent whole-brain radiation and SRS (25 Gy). Her follow-up MRIs in 2019 showed stable size of all lesions, but some areas were compatible with radiation necrosis in the left frontal and bilateral parietal lobes. A further follow-up MRI in January 2021 showed that the right temporal lesion had a 17 mm x 18 mm focus of hemorrhage. The area of enhancement of the right temporal lobe had increased in size and measured 18×13×21 mm, with a possible pseudo-progression related to hemorrhage inside it. A follow-up brain MRI after 3 weeks showed absorption of bleeding and stable size of the lesion.
She presented with headache, nausea, and vomiting 6 months later. Her brain MRI showed a significant increase in the size of the right temporal lesion and peri-lesional edema, with a significant midline shift (Figure 1A–1C). The enhancing part of the lesion was a 38×3×21-mm mass (blue arrow), which was much larger than on the previous MRI (Figure 1A). The patient was taken to surgery based on a probable preoperative diagnosis of recurrent metastatic disease. A right temporal craniotomy was performed. The dura was incised and, using the navigation system, the tumor was localized at the depth of 1 cm and we were able to reach the lesion. It was hard and well-defined and we could easily define and separate it from the surrounding swollen brain. Intraoperatively, it looked like a metastatic tumor and the lesion was totally resected grossly (Figure 1D–1F). Interestingly, the pathology was reported as intravascular papillary endothelial hyperplasia (Figures 2A–2C, 3A, 3B).
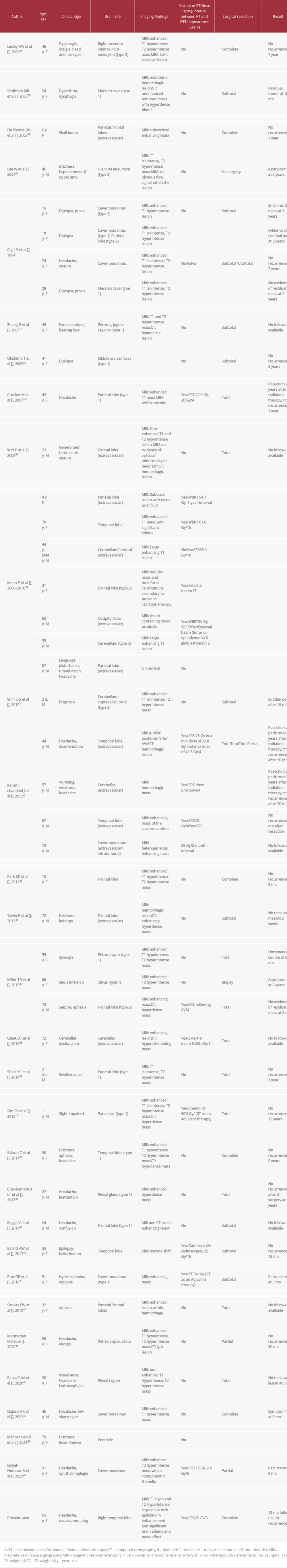
Postoperatively, the patient recovered very well and, considering the benign nature and gross total resection of the lesion, no further treatment was recommended. She received follow-up MRIs at 3, 6, and 12 months, which did not show any evidence of recurrence, and she has remained clinically asymptomatic. Table 1 summarizes previously reported cases of IPEH, and 44 cases have been reported before our case.
Discussion
Intravascular papillary endothelial hyperplasia (IPEH), also called Masson’s tumor, is a rare benign vascular lesion that consists of a hyperplastic monolayer of endothelial cells covering a bundle of hyalinized papillary vegetation [1,8,15,16]. IPEH is quite uncommon intracranially [1,8,9,17–19]; however, extracranial IPEH has recently been discovered in a variety of locations, including the limbs, mostly the feet and hands, the orbits, lips, and salivary glands. It can also be identified in vital organs such as the heart, lung, liver, and kidney, posing a life-threatening hazard to the patient.
IPEH seems to be more common in women than in men (57% female versus 43% male in the current literature review) and it is not limited to any age group (Table 1). According to reports, the number of men with IPEH is increasing. As a result, the findings support the idea that IPEH is gender-neutral. IPEH has been shown to be congenital in some studies. Shih et al [7] described a 2-day-old boy who had IPEH. On the other hand, no established inheritance pattern exists. The pathogenesis of IPEH is not fully understood. It can be primary or a complication of previous external beam radiotherapy or stereotactic radiosurgery (eg, using a gamma knife for treatment of arteriovenous malformation) [14]. In the current review of literature, 31% of patients with IPEH had a history of previous external beam radiotherapy or stereotactic radiosurgery. The average time interval between radiotherapy and onset of IPEH was 7.5 years (range, 2 months to 15 years) in these published cases. In 2 cases, radiotherapy was used as adjuvant therapy after resection of the lesion. In our presented case, radiosurgery was performed about 5 years before the presentation. There have been many reports on the long-term effect of radiotherapy on the CNS. Atherosclerotic changes, radiation necrosis, and development of vascular abnormalities, including cavernoma or aneurysms, are among the reported complications.
Moreover, intracranial IPEH can occur in combination with pre-existing vascular malformations, aneurysms, phlebectasia, lymphangiomas, thrombi, and angiokeratomas, without any history of radiotherapy [17,20]. Anti-angiogenic factors like angiostatin, thrombospondins (TSP), and endostatin, as well as hormonal variables, should be considered in the pathogenesis of IPEH [21]. Overall, according to reports, there are 3 types of IPEH [22]:A pure (primary) form that takes up residence in a normal blood vessel (usually a vein);A mixed (secondary) form that occurs in the presence of a prior cerebral varix, such as an AVM or hemangioma, which can result in intraventricular or intracerebral hemorrhage; andA rare extravascular form, which arises in an organized hematoma.
In the current case, the patient had a history of ovarian cancer and had a small enhancing lesion in her right temporal lobe in 2016 and underwent radiosurgery (up to 25 Gy) and whole-brain radiation therapy for multiple other lesions. She developed a small right temporal hemorrhage in January 2021 and a subsequent progressively enlarging mass in June 2021, which was finally resected, and pathology showed IPEH, not ovarian cancer. Therefore, in our case, IPEH developed in an organized clot, but the process may have been initiated by previous radiotherapy or focused radiosurgery by forming a cavernous malformation. Cavernomas are characterized by frequent micro-bleeding and subsequent micro-clot formation, and her IPEH developed inside this clot.
IPEH can develop in the base of the skull, the skull’s intravascular space, or the brain parenchyma. Depending on the location of the mass, they can present with increased intracranial pressure, compression of surrounding neurovascular structures, intracranial hemorrhage, and cranial nerve palsy [1,7,16,23]. In some cases, such as the presented case, it can manifest with a large mass, edema, and midline shift, which, if not treated in time, can lead to brain herniation and death.
Histologically, an IPEH is a solid pinky or red mass with vascularization [8]. IPEHs have no necrosis, atypia, increased mitotic figures, or any other obvious invasive behavior, which is one of their histopathological hallmarks [3]. Diaz-Flores et al [24] proposed that intussusceptive angiogenesis (IA) plays a key role in the histogenesis of IPEH. In another comparable investigation, they discovered that the creation of myriad pillars, especially in veins, is the cornerstone of IPEH. Akdur et al [25] examined IPEH histomorphological and immunohistochemical factors such as CD31, CD34, CD105, ki-67 staining, FVIII, and type IV collagen, in which CD31 and CD34 were found to be the most sensitive variables.
The differential diagnosis includes angiosarcoma, which is characterized by numerous irregularly shaped anastomosing vascular channels lined by atypical endothelial cells with a highly infiltrative architecture and poor demarcation. Compared to Masson tumors, angiosarcoma is typically covered by multilayers of plump endothelial cells with pleomorphism and mitotic activities [1–4,7–19,26].
Radiographically, it is difficult to differentiate an IPEH from a malignancy. There is no definite image characteristic for IPEH, which can appear as isointense to hypointense on T1, and hyperintense on T2, with varying degrees of contrast enhancement. It can cause severe peri-lesional edema and swelling of the brain, even with a significant mass effect. Overall, in most cases, it appears to be a malignant tumor on imaging, but it is not. Masson’s tumor can even be mistaken for a metastatic tumor clinically and radiographically, as it was in our case. However, it lacks critical malignant characteristics such as infiltration, necrosis, and nuclear pleomorphism on pathologic examinations. Therefore, clinical and radiological criteria are not sufficient for making a diagnosis, and pathological examination is necessary for a definite diagnosis.
IPEH can be treated with complete surgical removal; however, this may not be possible in all intracranial lesions, especially deeply seated lesions. The major challenge in the resection of these lesions is their location when in eloquent areas, avoiding damage to the surrounding swollen brain, and not causing more neurological deficits. Intraoperative neuronavigation and neuromonitoring will help to localize the lesion and minimize complications. Regardless of resection of the lesion, peri-lesional edema can persist or even worsen after the surgery. Therefore, dexamethasone should be continued postoperatively for a couple of days and tapered off slowly to treat brain edema. Perioperative seizure should be also prevented by antiseizure medications.
Small and deeply seated lesions in the highly eloquent areas of the brain and brain stem should not be treated surgically, as the morbidity and mortality may be worse that that caused by the lesion itself. Hence, gamma knife radiosurgery, chemotherapy, and stereotactic radiation have all been advised in circumstances where the risks of surgery and total resection as a primary treatment for the lesion outweigh the advantages [10,27]. Embolization before surgery has also been recommended in some cases to reduce bleeding during the procedure [8,19], although in our case the lesion was very well-circumscribed and bleeding was minimal. In cases of subtotal resection, anti-angiogenesis agents may be considered and patients should be closely monitored and followed by MRI [26].
Despite its benign histological characteristics, IPEH has a high non-neoplastic proliferation rate and a well-documented proclivity for recurrence after subtotal or even total resection [3]. Therefore, we should monitor these patients with an interval follow-up MRI and check for recurrence after removing the mass. In our case, we performed 3-, 6-, and 12-month follow-up MRIs; we found no evidence of recurrence, and the patient remained clinically well. Based on these findings, more prospective and multicenter studies are required to prove the relationship between radiation therapy and IPEH development.
Conclusions
IPEH is a non-cancerous vascular lesion that is difficult to diagnose before surgery. Despite its rarity, the possibility of Masson’s tumor should be considered in patients who have had a spontaneous hemorrhage without a history of any abnormalities such as cancer, and it also should be considered in the differential diagnosis of cerebral lesions with radiographic characteristics resembling vascular lesions. It has been associated with prior radiotherapy, which can be either radiosurgery or only external beam radiation. When feasible, IPEH can be cured by total surgical excision with the minimal risk of recurrence and this is the criterion standard treatment. Nevertheless, the probability of local progression with incomplete removal necessitates adjuvant therapy such as radiation, anti-angiogenesis agents, chemotherapy, and finally, regular follow-up imaging.
Figures
References:
1.. Cagli S, Oktar N, Dalbasti T, Intravascular papillary endothelial hyper-plasia of the central nervous system – four case reports: Neurol Med Chir (Tokyo), 2004; 44(6); 302-10
2.. Enzinger F, Enzinger WS, Weiss’s soft tissue tumors: Shock, 2008; 30(6); 754
3.. Prat GP, Jimenez MS, Caro PC, Case report and review of the literature: World Neurosurgery, 2018; 114; 194-203
4.. Karamchandani J, Vogel H, Fischbein N, Extravascular papillary endothelial hyperplasia mimicking neoplasm after radiosurgery: Case report: Neurosurgery, 2012; 70(4); E1043-48
5.. Masson P, Hemangioendotheliome vegetant intravasculaire: Bull Soc Anat (Paris), 1923; 93; 517
6.. Wehbé MA, Otto NR, Intravascular papillary endothelial hyperplasia in the hand: J Hand Surg, 1986; 11(2); 275-79
7.. Shih C-S, Burgett R, Bonnin J, Boaz J, Ho CY, Intracranial Masson tumor: Case report and literature review: J Neurooncol, 2012; 108(1); 211-17
8.. Avellino AM, Grant GA, Harris AB, Recurrent intracranial Masson’s vegetant intravascular hemangioendothelioma: Case report and review of the literature: J Neurosurg, 1999; 91(2); 308-12
9.. Baylor JE, Antonelli PJ, Rojiani A, Mancuso AA, Facial palsy from Masson’s vegetant intravascular hemangioendothelioma: Ear Nose Throat J, 1998; 77(5); 408-17
10.. Kristof RA, Van Roost D, Wolf HK, Schramm J, Intravascular papillary endothelial hyperplasia of the sellar region: Report of three cases and review of the literature: J Neurosurg, 1997; 86(3); 558-63
11.. Hashimoto H, Daimaru Y, Enjoji M, Intravascular papillary endothelial hyperplasia. A clinicopathologic study of 91 cases: Am J Dermatopathol, 1983; 5(6); 539-46
12.. Salyer WR, Salyer DC, Intravascular angiomatosis: Development and distinction from angiosarcoma: Cancer, 1975; 36(3); 995-1001
13.. Lee W, Hui F, Sitoh Y, Intravascular papillary endothelial hyperplasia in an intracranial thrombosed aneurysm: 3T magnetic resonance imaging and angiographical features: Singapore Med J, 2004; 45; 330-33
14.. Crocker M, Desouza R, Epaliyanage P, Masson’s tumour in the right parietal lobe after stereotactic radiosurgery for cerebellar AVM: Case report and review: Clin Neurol Neurosurg, 2007; 109(9); 811-15
15.. Duong D, Scoones D, Bates D, Sengupta R, Multiple intracerebral intravascular papillary endothelial hyperplasia: Acta Neurochir (Wien), 1997; 139(9); 883-86
16.. Sim SY, Lim YC, Won KS, Cho KG, Thirteen-year follow-up of parasellar intravascular papillary endothelial hyperplasia successfully treated by surgical excision: case report: J Neurosurg Pediatr, 2015; 15(4); 384-91
17.. Lesley WS, Kupsky WJ, Guthikonda M, Intravascular papillary endothelial hyperplasia arising within a posteroinferior cerebellar artery aneurysm: Case report and review of the literature: Neurosurgery, 2000; 47(4); 961-66
18.. Stoffman MR, Kim JH, Masson’s vegetant hemangioendothelioma: Case report and literature review: J Neurooncol, 2003; 61(1); 17-22
19.. Zhang R, Zhou L-F, Mao Y, Wang Y, Papillary endothelial hyperplasia (Masson tumor) of the petrous and jugulare region: Case report and literature review: Surg Neurol, 2005; 64(1); 55-60
20.. Requena L, Sangueza OP, Cutaneous vascular proliferations. Part II. Hyperplasias and benign neoplasms: J Am Acad Dermatol, 1997; 37(6); 887-922
21.. Tsoitis G, Milios G, Mandinaos C, [Vegetant intravascular hemangioendothelioma of the skin (cutaneous intravascular papillary endothelial hyperplasia)]: Ann Dermatol Venerol, 1991; 118(11); 866-68 [in French
22.. Znati K, Daoudi A, Chbani L, [Intravascular papillary endothelial hyper-plasia of the ankle: a case report]: Ann Chir Plast Esthet, 2009; 54(6); 600-2 [in French]
23.. Hagiwara A, Inoue Y, Shakudo M, Intracranial papillary endothelial hyperplasia: Occurrence of a case after surgery and radiosurgery: J Comput Assist Tomogr, 1999; 23(5); 781-85
24.. Díaz-Flores L, Gutiérrez R, González-Gómez M, Myriad pillars formed by intussusceptive angiogenesis as the basis of intravascular papillary endothelial hyperplasia (IPEH). IPEH is intussusceptive angiogenesis made a lesion: Histol Histopathol, 2021; 36(2); 217-28
25.. Akdur NC, Donmez M, Gozel S, Intravascular papillary endothelial hyperplasia: Histomorphological and immunohistochemical features: Diagn Pathol, 2013; 8(1); 167
26.. Lakka SS, Rao JS, Antiangiogenic therapy in brain tumors: Expert Rev Neurother, 2008; 8(10); 1457-73
27.. Miller TR, Mohan S, Tondon R, Intravascular papillary endothelial hyperplasia of the skull base and intracranial compartment: Clin Neurol Neurosurg, 2013; 115(10); 2264-67
28.. Lesley WS, Kupsky WJ, Guthikonda M, Intravascular papillary endothelial hyperplasia arising within a posteroinferior cerebellar artery aneurysm: Case report and review of the literature: Neurosurgery, 2000; 47(4); 961-65 ; discussion 966
29.. Stoffman MR, Kim JH, Masson’s vegetant hemangioendothelioma: Case report and literature review: J Neurooncol, 2003; 61(1); 17-22
30.. du Plessis DG, Balamurali G, Smith ET, Papillary endothelial hyperplasia associated with cortical dysplasia: Acta Neuropathol, 2003; 105(3); 303-8
31.. Lee W, Hui F, Sitoh YY, Intravascular papillary endothelial hyperplasia in an intracranial thrombosed aneurysm: 3T magnetic resonance imaging and angiographical features: Singapore Med J, 2004; 45(7); 330-33
32.. Ohshima T, Ogura K, Nakayashiki N, Tachibana E, Intravascular papillary endothelial hyperplasia at the superior orbital fissure: Report of a case successfully treated with gamma knife radiosurgery: Surg Neurol, 2005; 64(3); 266-69
33.. Witt P, Gault J, Kleinschmidt‐DeMasters BK, 23‐year‐old hispanic male with new onset seizures: Brain Pathology, 2008; 18(4); 594
34.. Mann P, Kleinschmidt-DeMasters BK, CNS Masson tumors: Am J Surg Pathol, 2016; 40(1); 81-93
35.. Park KK, Won YS, Yang JY, Intravascular papillary endothelial hyperplasia (Masson tumor) of the skull: Case report and literature review: J Korean Neurosurg Soc, 2012; 52(1); 52
36.. Taiwo F, Cowley P, Shieff C, A case of florid intracerebral reaction to haematoma mimicking a tumour: Br J Neurosurg, 2013; 27(1); 100-1
37.. Miller TR, Mohan S, Tondon R, Intravascular papillary endothelial hyperplasia of the skull base and intracranial compartment: Clin Neurol Neurosurg, 2013; 115(10); 2264-67
38.. Ginat DT, Walcott BP, Mordes D, Intracranial organizing hematoma with papillary endothelial hyperplasia features after resection and involved field radiotherapy for cerebellar juvenile pilocytic astrocytoma: Clin Imaging, 2014; 38(3); 322-25
39.. Shah HC, Mittal DH, Shah JK, Intravascular papillary endothelial hyperplasia (Masson’s tumor) of the scalp with intracranial extension: J Pediatr Neurosci, 2014; 9(3); 260-62
40.. Salaud C, Loussouarn D, Buffenoir K, Riem T, Masson’s tumor revealed by an intracerebral hematoma: Case report and a review of the literature. Neurochirurgie, 2017; 63(4); 327-29
41.. Charalambous LT, Penumaka A, Komisarow JM, Masson’s tumor of the pineal region: Case report: J Neurosurg, 2017; 128(6); 1725-30
42.. Bagga V, Kailaya-Vasan A, Wharton SB, Patel U, Intracerebral Masson’s tumor – slow-filling vascular lesion demonstrated by indocyanine green video angiography: World Neurosurg, 2017; 101; 812 e15–e19
43.. Barritt AW, Merve A, Epaliyanage P, Aram J, Intracranial papillary endothelial hyperplasia (Masson’s tumour) following gamma knife radiosurgery for temporal lobe epilepsy: Pract Neurol, 2017; 17(3); 214-17
44.. Sankey EW, Hynes JS, Komisarow JM, Masson’s tumor presenting as a left frontal intraparenchymal hemorrhage resulting in severe expressive aphasia during pregnancy: Case report: J Neurosurg, 2019; 134(1); 189-96
45.. Mezmezian MB, Arakaki N, Fallaza Moya S, Petroclival intravascular papillary endothelial hyperplasia with psammoma body‐like structures: Neuropathology, 2020; 40(3); 268-74
46.. Retzlaff AA, Arispe K, Cochran EJ, Zwagerman NT, Intravascular papillary endothelial hyperplasia of the pineal region: A case report and review of the literature: World Neurosurg, 2020; 133; 308-13
47.. Gajaria PK, Shenoy AS, Baste BD, Goel NA, Intravascular papillary endothelial hyperplasia of the cavernous sinus – a rare occurrence: Indian J Pathol Microbiol, 2021; 64(3); 541-44
48.. Manoranjan B, Mann JA, Joseph JT, Kelly JJ, Intraventricular Masson tumor: Case report and systematic review of primary intracranial intravascular papillary endothelial hyperplasia: J Neurosurg Sci, 2022; 66(5); 420-24
49.. Goyal-Honavar A, Balakrishnan R, Chacko G, Chacko AG, Radiation-induced intravascular papillary endothelial hyperplasia in a cavernous sinus hemangioma: Neurol India, 2022; 70(1); 359-62
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250