22 October 2020: Articles 
An Autopsy Case of an Elderly Patient with Classic Hodgkin Lymphoma Presenting with a Plethora of Clinical Symptoms and Signs
Unusual clinical course, Mistake in diagnosis
Hiroshi Kobayashi1ABDEFG*, Ryouya Seki2BDE, Masuo Ujita3BDE, Kana Hirayama4BCD, Satoshi Yamada5BD, Riuko Ohashi6BD, Yoshiro Otsuki7BE, Takuya Watanabe8CDE, Tadashi Yoshino9CDFDOI: 10.12659/AJCR.926177
Am J Case Rep 2020; 21:e926177
Abstract
BACKGROUND: Hodgkin lymphoma (HL) is a potentially curable disease with favorable outcomes. However, elderly patients with HL usually have more adverse prognostic factors and hence a much worse prognosis than younger patients.
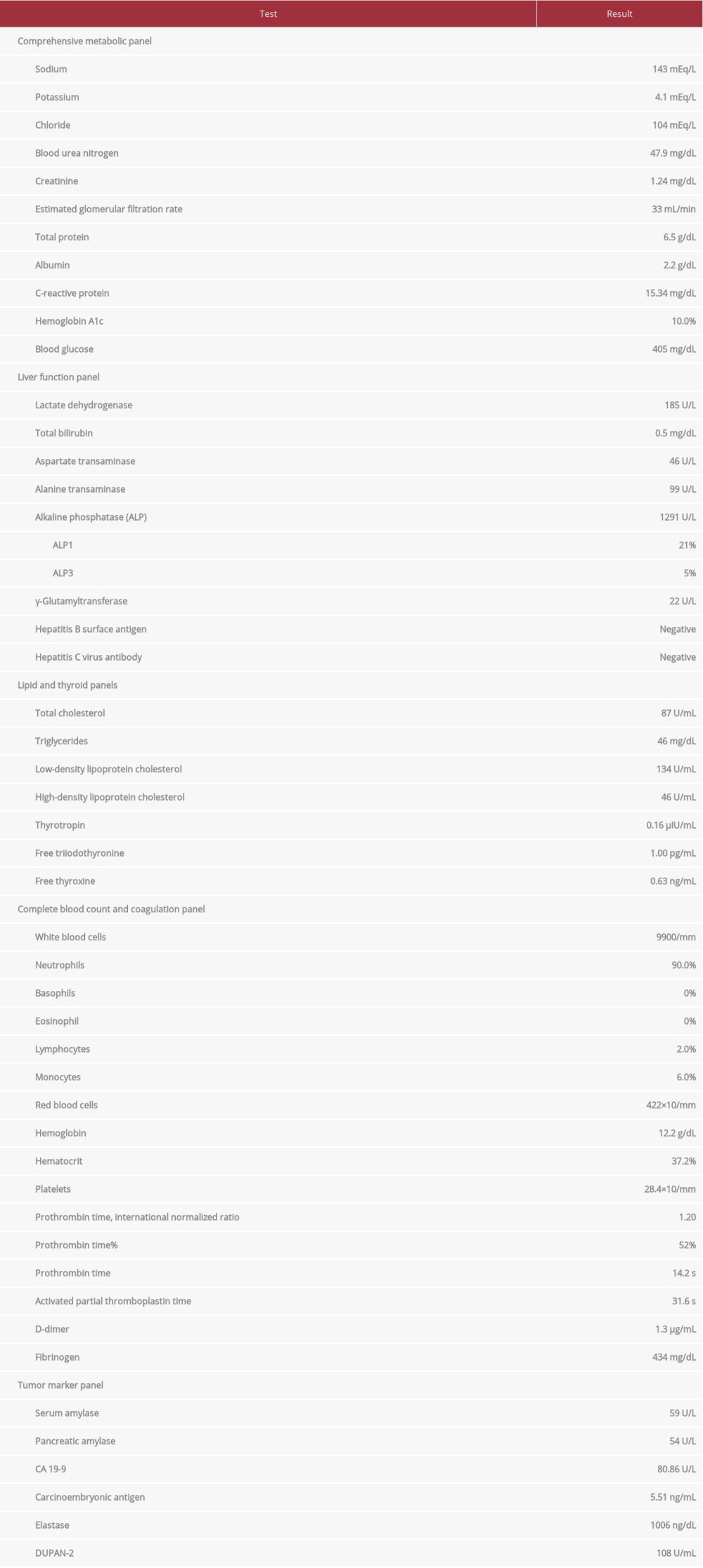
CASE REPORT: The patient was a woman in her 80s. She reported high fever, anorexia, and a weight loss of 8 kg within 5 months. She had been on treatment for diabetes mellitus and hypertension. She had undergone percutaneous coronary intervention and pacemaker implantation to treat acute coronary syndrome and sinus arrhythmia, respectively. Blood tests showed elevation of alkaline phosphatase, C-reactive protein, leukocyte count, CA 19-9, and carcinoembryonic antigen. Computed tomography did not show tumors in the liver, and cholangitis and sepsis were suspected. Aspartate transaminase, alanine aminotransferase, and total bilirubin gradually increased through the course of the patient’s hospital stay. Despite treatment, her condition deteriorated and she died 22 days after hospital admission. At autopsy, we found stage IV HL with lymph node swelling on both sides of the diaphragm, as well as diffusely disseminated nodules in the liver and spleen.
CONCLUSIONS: Our patient had several poor prognostic factors including B symptoms, comorbidity, advanced stage, Epstein-Barr virus infection, and expression of programmed death-ligand 1 and interleukin-6, all of which were closely connected with her advanced age. Her age and comorbidities may have been the most adverse prognostic factors for her illness. An effective HL screening method for elderly individuals should be developed to ameliorate poor prognosis and adverse outcomes.
Keywords: Antigens, CD274, Epstein-Barr Virus Infections, Hodgkin Disease, Interleukin-6, Alanine Transaminase, Autopsy, Herpesvirus 4, Human
Background
Hodgkin lymphomas (HLs) are lymphoid neoplasias that usually affect lymph nodes. Although primary extranodal involvement is rare, dissemination to more than one organ, such as the liver, bone marrow, or lungs, is associated with advanced (stage IV) disease [1]. Owing to advances in chemotherapy and radiation therapy, outcomes for patients with early-stage HL are excellent, and prognosis in advanced-stage disease is also very good [2–4].
Nevertheless, the 5-year survival rate of elderly patients (60 years or older) is much worse than that of younger patients (15 to 39 years old) [5,6]. In elderly individuals, HLs are characterized by aggressive disease and unfavorable prognostic features including advanced age, advanced stage, comorbidity, histological sub-type, Epstein-Barr virus (EBV) positivity, the number of macrophages and programmed death-ligand 1 (PD-L1)-positive cells, and serum levels of interleukin (IL)-6 [7–11]. Thus, the choice of treatment for HL in the elderly is a major challenge [4,7].
Despite a low rate of autopsies, a few recent reports describe HL cases that were diagnosed after death [12–14]. An autopsy study for unrecognized HL revealed a number of unfavorable prognostic factors that are common findings among patients who died of HL [15]. We found advanced HL at autopsy in an elderly patient in whom acute cholangitis and sepsis had been suspected. The patient had also exhibited worsening diabetes mellites and an elevation of tumor markers such as CA19-9 and carcinoembryonic antigen (CEA). This case had almost all the adverse prognostic factors mentioned above.
In this report, we discuss the prognostic factors that may have adversely affected the course of the patient. We also discuss the pathophysiological relation of HL with worsening diabetes mellites and suspected cholangitis. HL is a rather common, but vicious disease in elderly individuals [7,11]. However, screening methods to identify elderly patients with HL are lacking [16–18]. A new modality of the effective screening method is needed to ameliorate poor prognosis and adverse outcomes for elderly individuals with HL.
Case Report
AUTOPSY FINDINGS:
Macroscopic inspection revealed many swollen lymph nodes up to 3×3 cm in size in the mediastinal, retroperitoneal, hepatic hilar, and peripancreatic regions. These lymph nodes were grayish white in color and had an elastic consistency. The inspection also detected numerous white to brownish white nodules in the liver (915 g) and spleen (151 g) that were a few millimeters in size (Figure 3A, 3B). Both lungs had more than a dozen nodules of several millimeters in size (left: 297 g, right: 420 g). The right kidney had 2 nodules (7×7 mm) in the cortex, while the left kidney had no tumors. There was an elevated mucosal tumor (2×3 cm) at the base of the gall bladder. We did not observe any tumors or nodules in the pancreas. The pacemaker terminal was implanted in the right ventricle, and the stent was in the anterior descending branch of the left coronary artery. All 3 main branches of the coronary artery had severe atherosclerosis, with 65% to 90% occlusion of the lumens. Cut sections of the heart (285 g) showed an infarction scar (5.5×1.0 cm) in the posterolateral area of the left ventricle and a few smaller scars affecting the septum and anterior area. Yellow and translucent anasarca was seen: ascites of 700 mL, pleural effusions of 350 mL (left) and 300 mL (right), and pericardial effusion of 60 mL. Mild to moderate broncho-pneumonia was observed in the lungs.
Histology of the lymph nodes, liver, spleen, lungs, right kidney, and bone marrow similarly demonstrated scattered infiltration of atypical large cells with a dense admixture of inflammatory cells including small lymphocytes, plasma cells, and macrophages (Figure 4A). The atypical cells had large single nuclei or multiple nuclei with pale chromatin and prominent eosinophilic nucleolus. Neither geographical necroses nor angiocentric or angiodestructive lesions were seen. Immunohistochemically, the atypical cells were diffusely positive for CD15, CD30, and Mum1 (Figure 4B–4D); focally positive for CD138; and negative for CD3, CD4, CD8, CD20, and CD79a. We diagnosed the case as mixed-cellularity classical HL (MCCHL). We performed immunostaining with PAX5 (Leica 1EW) twice and could not obtain the proper immunohistochemical results owing to no positive staining of Hodgkin and Reed/Sternberg (HRS) cells and the background lymphocytes.
In situ hybridization analysis with Epstein-Barr virus-encoded RNA (EBER; Leica BOND EBER probe) showed strong positivity for many HRS cells (Figure 5A). Many macrophages in the tumor microenvironment were positive for CD-68 (Agilent KP1; Figure 5B). HRS cells positively stained strongly for PD-L1 (Ventana SP142; Figure 5C) and IL-6 (Leica 10C12; Figure 5D), moderately for LMP1 (Dako CS.1–4) and granzyme B (Leica 11F1), and weakly for tumor necrosis factor (TNF)-α (Abcam P/T2). A large number of the inflammatory cells were also positive for PD-L1 and IL-6 (Figure 5C, 5D). Monoclonal or oligoclonal proliferation of plasma cells was not seen with immunohisto-chemistry of kappa and lambda light chains of immunoglobulin.
In the liver, HRS cells and the reactive components heavily infiltrated the portal tracts (Figure 6A). Many hepatic bile ducts were positive for CA 19-9 (Figure 6B) but not for CEA. The gall bladder tumor was histologically identified as adenoma with severe dysplasia/carcinoma in situ, and the neoplastic cells were immunohistochemically positive for CEA.
Discussion
In a surveillance study with more than 21 500 HL cases, multivariate analyses suggested that adverse prognostic factors included age (>19 years old), histologic subtypes (mixed cellularity and lymphocyte-depleted), stage IV, presence of B symptoms, no reported use of radiation, and non-white race [19]. In the International Prognostic Score, only age (≥45 years) and hemoglobin level retained independent significance in multivariate analyses of advanced-stage HL [20]. Elderly patients (≥60 years of age) with HL have disproportionately worse survival rate compared with younger patients [21]. Net survival gradually decreases with age, and the 5-year survival is the highest (over 90%) in the youngest individuals (15 to 39 years), approximately 50% in patients aged 70 to 79 years, and less than 30% in those aged 80 years or older [5]. Accordingly, age is considered as one of the most important prognostic factors in HL, and it is intrinsically associated with HL biology [22].
According to van Sporsen et al. [23], 21% of HL patients younger than 60 years have serious comorbid conditions compared with 58% of the older patients. Comorbid conditions chiefly comprise cardiovascular disease, hypertension, chronic obstructive pulmonary disease, and diabetes. In multivariate analyses of elderly HL patients, comorbidity, but not age (60 or older), was regarded as a significant factor of overall survival, whereas the presence of a comorbidity, stage, and presence of B symptoms were each independently correlated with inferior survival [24]. The presence of comorbidity as an independent prognostic factor is particularly relevant for older patients. Moreover, the presence of severe comorbidity may distract the patient, clinician, or both, such that the early symptoms of tumor growth may go unnoticed, leading to delayed diagnosis [25]. Our patient had actually undergone percutaneous coronary intervention and pacemaker implantation for acute coronary syndrome and sinus arrhythmia, respectively, 7 months earlier. She did not present with any symptoms and signs of HL at that time. In addition, there is no true consensus for treatment of elderly patients with HL, primarily owing to intolerance of curative treatment caused by comorbidities. Therefore, the choice of treatment is a major challenge [4,7].
It has been recognized that the number of tumor-infiltrating CD68+ and CD163+ macrophages constitutes a major biomarker that is predictive of inferior progression-free and overall survival [8,26]. Several studies have confirmed this observation [27–29]. CD68 and CD163 expression in classical HL (CHL) is related to increased age, EBER positivity, and MCCHL [28]. Old age is associated with a high frequency of presence of EBV and MCCHL [22,30]. Immunosenescence may be an explanatory factor for the presence of EBV in older patients [22,30]. MCCHL has a close relationship with EBV infection [1], and older patients with EBV-positive HL have significantly poorer outcomes compared with those with EBV-negative HL [30,31].
The natural function of signaling between PD-1 and PD-L1 is to limit certain T-cell-mediated immune responses. T-cell exhaustion is essential to the pathogenesis of HL [32,33]. Nonetheless, PD-L1 and PD-L2 on HRS cells have no prognostic significance [34]. In several tumor types, especially CHL and other tumors with a marked inflammatory infiltrate, a major component of PD-L1 expression within the total cellularity is supposed to derive from tumor-infiltrating macrophages [9,33,35]. Thus, in addition to the CD68 and CD163 expression, the number of PD-L1-positive cells in formalin-fixed paraffin-embedded tissue can be considered as an adverse prognostic factor for elderly patients with HL [9,36].
IL-6 expression in HRS cells, as detected by immunohistochemistry, correlates with B symptoms [10,37]. The expression in background cells independently predicts poor response and freedom from treatment failure in pediatric HL [38]. Although we could not examine serum IL-6, we believe that it was probably elevated in the serum because HRS cells and many inflammatory cells immunohistochemically showed strong positivity for IL-6. The serum level of IL-6 as well as those of IL-1 and TNF-α offers an unfavorable prognosis [10,39]. In addition, patients with increased IL-6 and IL-2R have a significantly higher risk of early relapse and death [10]. However, further studies are needed to clarify virus-associated cytokine effects on poor prognosis in elderly patients with HL [30].
On the other hand, the serum level of TNF-α or IL-6 increases when glucose tolerance is impaired in diabetics compared with nondiabetics [40–42]. Worsening of glucose control positively and linearly correlates with high levels of IL-6 and leptin [43]. There are a few reported cases with IL-6-producing tumors, including lung cancer and adrenal pheochromocytoma, that exacerbated glucose tolerance in diabetic or non-diabetic patients [44–46]. Worsening of glucose tolerance in our patient may have been caused by excess production of IL-6 by HRS cells and inflammatory cells. Nonetheless, the relationship between IL-6 production and abnormal glucose tolerance in HL patients remains to be unraveled with more cases.
While liver diseases occur as a hepatic manifestation of paraneoplastic syndrome in HL [47–49], the frequency of direct involvement with HL ranges from 2% to 20% at the time of diagnosis [1,50,51] and up to two-thirds of the cases at autopsy or necropsy [15,52]. The portal tracts are the most common site for HL involvement [52]. The involvement more typically manifests as miliary lesions (<1 cm) than as masses on CT [47]. Hence, enhanced CT and positron emission tomography (PET) imaging may be required to detect them. The lesions are more frequently seen in portal areas than in hepatic parenchyma in liver biopsy [53]. The histology of portal involvement sometimes suggests acute cholangitis and nonspecific large (≥1 mm) inflammatory infiltrates [53]. The frequency of liver involvement differs in subtypes of CHL. Lymphocyte-depleted CHL (LDCHL) has involvement of the bone marrow (11%) and liver (19%) significantly more often than the other subtypes [50]. Although LDCHL has a much poorer prognosis than the other subtypes, LDCHL and MCCHL are viewed as 2 grades of a single disease entity, often occurring in the setting of HIV infection and conferring a significantly worse prognosis [39].
Finally, HL is not a very rare disease among elderly individuals. The percentage of patients aged over 60 years ranges between 15% and 35% [7,11]. Cases in this population are characterized by aggressive disease and unfavorable prognostic features, which many authors have reported. An effective screening method to detect HL in elderly patients at an early stage and in younger patients is expected in the near future [17,18]. Less toxic and effective therapies are also needed [6,11], in addition to next-generation strategies to prevent and treat EBV infection as a means to control EBV-associated diseases [54–56]. These efforts will result in filling the disproportionately large gap between young and older patients in terms of the effectiveness of treatment and disease prognosis.
Conclusions
We reported an autopsy case of HL in an elderly woman. Such reports are very rare probably because of a low rate of autopsies. We suspected excessive production of IL-6 as the cause of worsening diabetes mellites in our case. Study of a larger number of cases is needed to unravel the relationship between IL-6 production and abnormal glucose tolerance in HL patients. HL may often involve the liver as miliary lesions occur along the portal tracts, which can be confused with cholangitis. Thus, enhanced CT and PET imaging may be required to detect them.
Elderly patients with HL usually have more adverse prognostic factors than younger patients. We discussed the adverse factors, including comorbidity, CD68+ macrophages, EBV infection, and PD-L1 and IL-6 expression in the cells of tumor microenvironment. All of these factors appear to be closely connected with advanced age and pose a major challenge in the clinical management of elderly patients. A new modality of screening for HL in elderly individuals is needed.
Figures
References:
1.. Swerdlow SH, Campo E, Harris NL: WHO classification of tumours of haematopoietic and lymphoid tissues, 2017, Lyon, IARC
2.. Stephen MA, Hodgkin lymphoma: Diagnosis and treatment: Mayo Clin Proc, 2015; 90(11); 1774-83
3.. Townsend W, Linch D, Hodgkin’s lymphoma: Lancet, 2012; 380; 836-47
4.. Makita S, Maruyama D, Miyagi Maeshima A, Clinical features and outcomes of 139 Japanese patients with Hodgkin lymphoma: Int J Hematol, 2016; 104(2); 236-44
5.. , Hodgkin lymphoma statistics [Internet] [Cited 2020 March 22]. https://www.cancerresearchuk.org/
6.. Björkholm M, Weibull CE, Eloranta S, Greater attention should be paid to developing therapies for elderly patients with Hodgkin lymphoma – a population-based study from Sweden: Eur J Haematol, 2018; 101; 106-14
7.. Thyss A, Saada E, Gastaud F, Hodgkin’s lymphoma in older patients: An orphan disease?: Mediterr J Hematol Infect Dis, 2014; 6; e2014050
8.. Steidel C, Lee T, Shah SP, Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma: N Eng J Med, 2010; 362; 875-85
9.. Mottok A, Steidl C, Biology of classical Hodgkin lymphoma: Implications for prognosis and novel therapies: Blood, 2018; 131; 1654-65
10.. Marri PR, Hodge LS, Maurer MJ, Prognostic significance of pretreatment serum cytokines in classical Hodgkin lymphoma: Clin Cancer Res, 2013; 19; 6812-19
11.. Böll B, Görgen H, The treatment of older Hodgkin lymphoma patients: Brit J Haematol, 2019; 184; 82-92
12.. Shah S, Smith M, Butler R, A case of Hodgkin lymphoma mimicking lymphomatoid granulomatosis diagnosed at autopsy: Lab Med, 2018; 49; 80-86
13.. Ichimata S, Kobayashi M, Ohya M, A fulminant case of classical Hodgkin lymphoma: A diagnostic dilemma of Epstein-Barr virus-positive large B-cell lymphomas: Pathol Int, 2019; 69; 407-13
14.. Di Pietra L, Gardiman M, Terranova C, Postpartum maternal death associated with undiagnosed Hodgkin’s lymphoma: Med Sci Law, 2012; 52; 174-77
15.. Hasle A, Mellemgaard A, Hodgkin’s disease diagnosed post mortem: A population based study: Br J Cancer, 1993; 67; 185-89
16.. Pinsky PF, Principles of cancer screening: Surg Clin North Am, 2015; 95; 953-66
17.. Kosaka N, Iguchi H, Ochiya T, Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis: Cancer Sci, 2010; 101; 2087-92
18.. Moia R, Favini C, Rasi S, Liquid biopsy in lymphomas: A potential tool for refining diagnosis and disease monitoring: J Cancer Metastasis Treat, 2019; 5; 67
19.. Bazzeh F, Rihani R, Howard S, Sultan I, Comparing adult and pediatric Hodgkin lymphoma in the surveillance, epidemiology and end results program, 1988–2005: An analysis of 21 734 cases: Leuk Lymphoma, 2010; 51(12); 2198-207
20.. Moccia AA, Donaldson J, Chhanabhai M, International prognostic score in advanced-stage Hodgkin’s lymphoma: Altered utility in the modern era: J Clin Oncol, 2012; 30(27); 3383-88
21.. Evens AM, Sweetenham JW, Horning SJ, Hodgkin lymphoma in older patients: An uncommon disease in need of study: Oncology (Williston Park), 2008; 22(12); 1369-79
22.. Cuccaoro A, Bartolomei F, Cupelli E, Prognostic factors in Hodgkin lymphoma: Mediterr J Hematol Infect Dis, 2014; 6; e2014053
23.. van Spronsen DJ, Janssen-Heijnen MLG, Lemmens VEPP, Independent prognostic effect of co-morbidity in lymphoma patients: Results of the population-based Endhoven Cancer Registry: Eur J Cancer, 2005; 41; 1051-57
24.. Galli E, Cuccaro A, Maiolo E, Comorbidity assessment to determine prognosis in older adult patients with classical Hodgkin lymphoma: Hematol Oncol, 2020; 38(2); 153-61
25.. Gurney J, Sarfati D, Stanley J, The impact on patient comorbidity on cancer stage at diagnosis: Br J Cancer, 2015; 113; 1375-80
26.. Tzankov A, Matter MS, Dirnhofer S, Refined prognostic role of CD68-positive tumor macrophages in the context of the cellular micromilieu of classical Hodgkin lymphoma: Pathobiology, 2010; 77; 301-8
27.. Kamper P, Bendix K, Hamilton-Dutoit S, Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma: Haematologica, 2011; 96; 269-76
28.. Tan KL, Scott DW, Hong F, Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: A correlative study from the E2496 Intergroup trial: Blood, 2012; 120; 3280-87
29.. Panico L, Ronconi F, Lepore M, Prognostic role of tumor-associated macrophages and angiogenesis in classical Hodgkin lymphoma: Leuk Lymphoma, 2013; 54; 2418-25
30.. Piris MA, Medeiros J, Chang K-C, Hodgkin lymphoma: A review of pathological features and recent advances in pathogenesis: Pathology, 2020; 52; 154-65
31.. Jarret RF, Stark GL, White J, Impact of tumor Epstein-Barr virus status on presenting features and outcome in aged-defined subgroups of patients with classic Hodgkin lymphoma: A population-based study: Blood, 2005; 106; 2444-51
32.. Yamamoto R, Nishikori M, Kitawaki T, PD-1–PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma: Blood, 2008; 111; 3220-24
33.. Carey CD, Gusenleitner D, Lipschitz M, Topological analysis reveals a PD-L1 associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma: Blood, 2017; 130; 2420-30
34.. Hollander P, Kamper P, Smedby KE, High proportions of PD-1+ and PD-L1 leukocytes in classical Hodgkin lymphoma microenvironment are associated with inferior outcome: Blood Adv, 2017; 1; 1427-39
35.. Chen BJ, Chapuy B, Ouyang J, PD-L1 expression is characteristic of a subset aggressive B-cell lymphomas and virus-associated malignancies: Clin Cancer Res, 2013; 19; 3462-73
36.. Hollander P, Amini R-M, Ginman B, Expression of PD-1 and PD-L1 increase in consecutive biopsies in patients with classical Hodgkin lymphoma: PLoS One, 2018; 13; e0204870
37.. Reynolds GM, Billingham LJ, Gray LJ, Interleukin 6 expression by Hodgkin/Reed-Sternberg cells is associated with the presence of ‘B’ symptoms and failure to achieve complete remission in patients with advanced Hodgkin’s disease: Br J Haematol, 2002; 118; 195-201
38.. Bhethanabhotla S, Tiwari A, Sharma MC, Prognostic significance of IL-6 in Hodgkin lymphoma: Ind J Pediatrics, 2019; 86; 551-54
39.. Mani H, Jaffe ES, Hodgkin lymphoma: An update on its biology with newer insights into classification: Clin Lymphoma Myeloma, 2009; 9; 206-16
40.. Müller S, Martin S, Koenig W, Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-α or its receptors: Diabetologica, 2002; 45; 805-12
41.. Rotter V, Nagaev I, Smith U, Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, over-expressed in human fat cells from insulin-resistant subjects: J Biol Chem, 2003; 278; 45777-84
42.. Nakamura M, Oda S, Sadahiro T, Correlation between high blood IL-6 level, hyperglycemia, and glucose control in septic patients: Critical Care, 2012; 16; R58
43.. Mirza S, Hossain M, Mathews C, Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican American: A cross-sectional study: Cytokine, 2012; 57; 136-42
44.. Kang JM, Lee WJ, Kim WB, Systemic inflammatory syndrome and hepatic inflammatory cell infiltration caused by an interleukin-6 producing pheochromocytoma: Endocr J, 2005; 52; 193-98
45.. Furukawa S, Fujiwara K, Omoto M, [A case of IL-6 producing pheochromocytoma with its secondary diabetes mellites being ameliorated by administrating naproxen prior to resection of the tumor]: Diabetes (Tounyoubyou), 2014; 57; 913-20 [in Japanese]
46.. Aoki C, Suzuki K, Nakano A, [A case of type 2 diabetes with early-stage lung cancer presenting with marked increase of blood glucose in association with high level of IL-6]: Int Med (Naika), 2010; 105; 548-52 [in Japanese]
47.. Barta SK, Yahalom J, Shia J, Hamlin PA, Idiopathic cholestasis as a paraneoplastic phenomenon in Hodgkin‘s lymphoma: Clin Lymphoma Myeloma, 2006; 7; 77-82
48.. Scalabrini DR, Caravelli D, Schianca FC, Complete remission of paraneoplastic vanishing bile duct syndrome after successful treatment of Hodgkin’s lymphoma: A case report and review of the literature: BMC Res Notes, 2014; 7; 529
49.. Man KM, Drejet A, Keeffe EB, Primary sclerosing cholangitis and Hodgkin’s disease: Hepatology, 1993; 18; 1127-31
50.. Klimm B, Franklin J, Stein H, Lymphocyte-depleted classical Hodgkin’s lymphoma: A comprehensive analysis from the German Hodgkin Study Group: J Clin Oncol, 2011; 29; 3914-20
51.. Guermazi A, Brice P, de Kervilier E, Extranodal Hodgkin disease: Spectrum of disease: Radiographics, 2001; 21; 161-79
52.. Levitan R, Diamond HD, Craver LF, The liver in Hodgkin’s disease: Gut, 1961; 2; 60-71
53.. Dich NH, Goodman ZD, Klein MA, Hepatic involvement in Hodgkin’s disease. Clues to histologic diagnosis: Cancer, 1989; 64; 2121-26
54.. van Zyl DG, Mautner J, Delecluse HJ, Progress in EBV vaccines: Front Oncol, 2019; 9; 104
55.. Rühl J, Leung CS, Münz C, Vaccination against the Epstein-Barr virus: Cell Mol Life Sci, 2020 [Online ahead of print]
56.. Andrei G, Trompet E, Snoeck R, Novel therapeutics for Epstein-Barr virus: Molecules, 2019; 24; 997
Figures
In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864