15 June 2021: Articles 
A Case of Rapid Transformation from Hydatidiform Mole to Invasive Mole: The Importance of β-hCG (Human Chorionic Gonadotropin) Serum Levels in Follow-Up Evaluation
Unusual setting of medical care, Rare disease, Educational Purpose (only if useful for a systematic review or synthesis)
Ali Budi Harsono1ABCDEFG, Yudi Mulyana Hidayat1CDG, Gatot Nyarumenteng Adhipurnawan Winarno1EF*, Aisyah Shofiatun Nisa1EF, Firas Farisi Alkaff2EFDOI: 10.12659/AJCR.931156
Am J Case Rep 2021; 22:e931156
Abstract
BACKGROUND: Gestational trophoblastic disease (GTD) is a spectrum of disorders consisting of premalignant (ie, complete [CHM] and partial hydatidiform moles [PHM]) and malignant conditions (ie, invasive moles, choriocarcinoma, placental site trophoblastic tumors, and epithelioid trophoblastic tumor). If GTD persists after initial treatment and has persistent elevated beta human chorionic gonadotropin (β-hCG), it is referred to as post-molar gestational trophoblastic neoplasia (pGTN). To date, there is no detailed information regarding how fast invasive moles can develop from CHM. However, the risk of developing any pGTN from CHM is rare within 1 month and is greatest in the first 12 months after evacuation, with most cases presenting within 6 months.
CASE REPORT: We present a case of a 46-year-old primigravida woman with rapid transformation of an invasive mole. In the beginning, the patient had a chief concern of a uterus size greater than the gestational dates. Laboratory evaluation showed high β-hCG serum level (>300 000 mIU/mL), and ultrasonography evaluation revealed a hydatidiform mole. Suction evacuation and curettage procedures were then performed. Pathology evaluation afterwards revealed a complete hydatidiform mole without any sign of malignancy. Twenty-two days afterwards, the patient came to the emergency room with vaginal bleeding. β-hCG serum level was high (53 969 mIU/mL), and ultrasonography examination showed the presence of fluid filling the uterine cavity. The patient was then diagnosed with GTN, and hysterectomy was chosen as the treatment of choice. After the surgery, her β-hCG serum level gradually reverted back to normal.
CONCLUSIONS: Invasive moles can develop less than 1 month after suction evacuation and curettage procedure for CHM. Serial β-hCG serum level evaluation according to the guideline should be performed to prevent late diagnosis, which could lead to the development of metastasis and worsen the prognosis.
Keywords: gestational trophoblastic disease, Hydatidiform Mole, Hydatidiform Mole, Invasive, Chorionic Gonadotropin, Follow-Up Studies, Placenta, Pregnancy, Uterine Neoplasms
Background
Gestational trophoblastic disease (GTD) is a spectrum of disorders consisting of premalignant (ie, complete [CHM] and partial hydatidiform mole [PHM]) and malignant conditions (ie, invasive mole, choriocarcinoma, placental site trophoblastic tumor, and epithelioid trophoblastic tumor). If GTD persists after initial treatment and has persistent elevated beta human chorionic gonadotropin (β-hCG), it is referred to as post-molar gestational trophoblastic neoplasia (pGTN) [1]. Studies showed that the incidence of PHM is higher than that of CHM, but CHM has a more than 7 times higher risk of developing pGTN [2,3]. About 10–17% will develop into an invasive mole, which is a benign tumor that arises from myometrial invasion via direct extension through tissue or venous channels. Although it is a benign tumor, the mortality rate is 15% [4].
To date, there is no detailed information regarding how fast an invasive mole can develop from CHM. However, the risk of developing any invasive mole from CHM is low within 1 month and is greatest in the first 12 months after evacuation, with most cases presenting within 6 months [3,5]. In this case report, we present a rare case of an invasive mole that developed 22 days after the evacuation of CHM. This case report highlights the importance of serial β-hCG follow-up evaluation, as recommended in the guidelines [6].
Case Report
A 46-year-old primigravida woman was referred to our outpatient clinic in October 2020 with a chief concern of a uterus size greater than the gestational dates. Her last date of menstruation was 8 weeks ago, and the pregnancy test showed a positive result. The patient never had a miscarriage or underwent an abortion. Vital signs were unremarkable. Physical evaluation revealed an enlarged uterus proportional to 16 weeks of pregnancy. Laboratory evaluation showed elevated β-hCG serum level (>300 000 mIU/mL), while other parameters were within normal ranges. Ultrasonography (USG) evaluation revealed a hydatidiform mole (Figure 1); therefore, suction evacuation and curettage were performed. After the procedures, USG reevaluation showed that the hydatidiform mole was completely removed (Figure 2). The samples were sent to the pathology anatomy lab for macroscopic and microscopic evaluation, which later revealed a complete hydatidiform mole without any sign of malignancy (Figures 3, 4).
The patient was scheduled for follow-up β-hCG serum level evaluation on the 7th, 14th, and 21st days after the procedure. However, the patient did not comply with the follow-up schedule. The patient came on the 9th day after the procedure, where follow-up evaluation showed that β-hCG serum levels fell to 9838 mIU/mL. Because of the significant reduction of the β-hCG serum levels, the patient decided to skip the evaluation on the 14th day. On the 22nd day, the patient came to the emergency room with severe vaginal bleeding. The USG examination showed the presence of fluid in the uterine cavity (Figure 5). The hemoglobin level had fallen to 6 mg/dL, and the β-hCG serum level drastically increased to 53 969 mIU/ mL. The patient was then diagnosed with GTN. The chest X-ray evaluation showed no sign of distant metastases.
Based on several considerations and discussion with the patient, hysterectomy was chosen as the treatment of choice. Hysterectomy was performed 2 days later, after the hemoglobin level was increased to 8 mg/dL. The specimen was then sent to the pathology anatomy lab for evaluation, which later established the diagnosis of an invasive mole (Figures 6, 7). After the surgery, the patient was scheduled for serial β-hCG serum level evaluation. The β-hCG serum level were 355 mIU/mL on the 7th day, 115 mIU/mL on the 14th day, and 62 mIU/mL on the 21st day.
Discussion
STUDY LIMITATIONS:
The patient did not comply with the follow-up schedule for serial β-hCG serum level evaluation. Therefore, we cannot provide data on exactly when the β-hCG level started to rise again.
Conclusions
Invasive moles can develop in less than 1 month after suction evacuation and curettage procedures for CHM. Serial βhCG serum level evaluation according to the latest guidelines should be performed to prevent late diagnosis, which could lead to the development of metastasis and worsen the prognosis. Further research is needed to understand the pathological mechanism of the transformation and factors that contribute to the rapidity of the transformation.
Figures
References:
1.. , Management of Gestational Trophoblastic Disease: BJOG, 2021; 128(3); e1-e27
2.. Seckl MJ, Sebire NJ, Berkowitz RS, Gestational trophoblastic disease: Lancet, 2010; 376(9742); 717-29
3.. Coyle C, Short D, Jackson L, What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients: Gynecol Oncol, 2018; 148(2); 254-57
4.. Lurain JR, Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole: Am J Obstet Gynecol, 2010; 203(6); 531-39
5.. Cavaliere A, Ermito S, Dinatale A, Pedata R, Management of molar pregnancy: J Prenat Med, 2009; 3(1); 15-17
6.. Ngan HYS, Seckl MJ, Berkowitz RS, Update on the diagnosis and management of gestational trophoblastic disease: Int J Gynecol Obstet, 2018; 143(S2); 79-85
7.. Zhou X, Chen Y, Li Y, Duan Z, Partial hydatidiform mole progression into invasive mole with lung metastasis following in vitro fertilization: Oncol Lett, 2012; 3(3); 659-61
8.. Martinez Leocadio C, Garcia Villayzan J, Garcia-Foncillas Lopez J, Invasive mole in a perimenopausal woman with lung and vaginal metastases: A case report: Clin Case Rep, 2019; 7(12); 2300-5
9.. Strohl AE, Lurain JR, Clinical epidemiology of gestational trophoblastic disease: Curr Obstet Gynecol Rep, 2014; 3(1); 40-43
10.. Tham BWL, Everard JE, Tidy JA, Gestational trophoblastic disease in the Asian population of Northern England and North Wales: BJOG, 2003; 110(6); 555-59
11.. Wang Q, Fu J, Hu L, Prophylactic chemotherapy for hydatidiform mole to prevent gestational trophoblastic neoplasia: Cochrane Database Syst Rev, 2017(9); CD007289
12.. Sebire NJ, Fisher RA, Foskett M, Risk of recurrent hydatidiform mole and subsequent pregnancy outcome following complete or partial hydatidiform molar pregnancy: BJOG, 2003; 110(1); 22-26
13.. Ngan H, Bender H, Benedet J, Gestational trophoblastic neoplasia, FIGO 2000 staging and classification: Int J Gynecol Obstet, 2003; 83; 175-77
14.. Wolfberg AJ, Berkowitz RS, Goldstein DP, Postevacuation hCG levels and risk of gestational trophoblastic neoplasia in women with complete molar pregnancy: Obstet Gynecol, 2006; 107(3); 743
15.. Arima T, Matsuda T, Takagi N, Wake N, Association of IGF2 and H19 imprinting with choriocarcinoma development: Cancer Genet Cytogenet, 1997; 93(1); 39-47
16.. Jun S-Y, Ro JY, Kim K-R, p57kip2 is useful in the classification and differential diagnosis of complete and partial hydatidiform moles: Histopathology, 2003; 43(1); 17-25
17.. Yazaki-Sun S, Daher S, de Souza Ishigai MM, Correlation of c-erbB-2 oncogene and p53 tumor suppressor gene with malignant transformation of hydatidiform mole: J Obstet Gynaecol Res, 2006; 32(3); 265-72
18.. Ståhle-Bäckdhal M, Inoue M, Zedenius J, Decreased expression of Ras GTPase activating protein in human trophoblastic tumors: Am J Pathol, 1995; 146(5); 1073
19.. Yuxia W, Yang C, Yongyu S, Expression of c-erbB2 in gestational trophoblastic disease and its clinical significance: J Huazhong Univ Sci Technolog Med Sci, 2002; 22(2); 123-25
20.. Wargasetia TL, Shahib N, Martaadisoebrata D, Characterization of apoptosis and autophagy through Bcl-2 and Beclin-1 immunoexpression in gestational trophoblastic disease: Iran J Reprod Med, 2015; 13(7); 413-20
21.. Khooei A, Pasdar FA, Fazel A, Expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins in hydatidiform moles and placentas with hydropic changes: Acta Medica Iranica, 2019; 27-32
22.. Shih I-M, Gestational trophoblastic neoplasia – pathogenesis and potential therapeutic targets: Lancet Oncol, 2007; 8(7); 642-50
23.. Olvera M, Harris S, Amezcua CA, Immunohistochemical expression of cell cycle proteins E2F-1, Cdk-2, Cyclin E, p27(kip1), and Ki-67 in normal placenta and gestational trophoblastic disease: Mod Pathol, 2001; 14(10); 1036-42
24.. Sebire NJ, Rees HC, Peston D, p57KIP2 immunohistochemical staining of gestational trophoblastic tumours does not identify the type of the causative pregnancy: Histopathology, 2004; 45(2); 135-41
25.. Yang X, Zhang Z, Jia C, The relationship between expression of c-ras, c-erbB-2, nm23, and p53 gene products and development of trophoblastic tumor and their predictive significance for the malignant transformation of complete hydatidiform mole: Gynecol Oncol, 2002; 85(3); 438-44
26.. Okamoto T, Niu R, Yamada S, Osawa M, Reduced expression of tissue inhibitor of metalloproteinase (TIMP)-2 in gestational trophoblastic diseases: Mol Hum Reprod, 2002; 8(4); 392-98
Figures
Tables
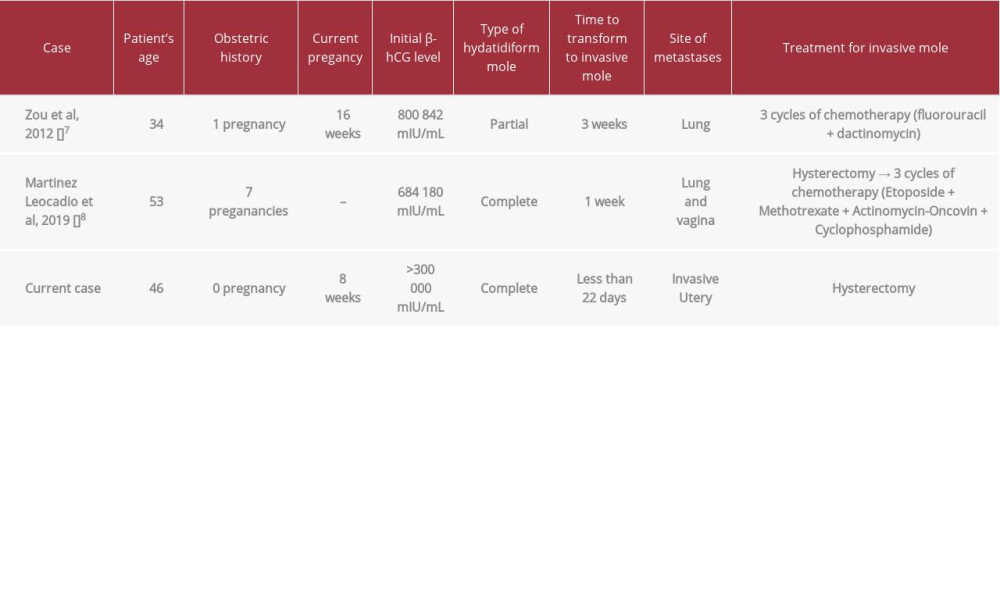 Table 1.. Reported cases of invasive mole rapid transformation after curettage procedure.
Table 1.. Reported cases of invasive mole rapid transformation after curettage procedure.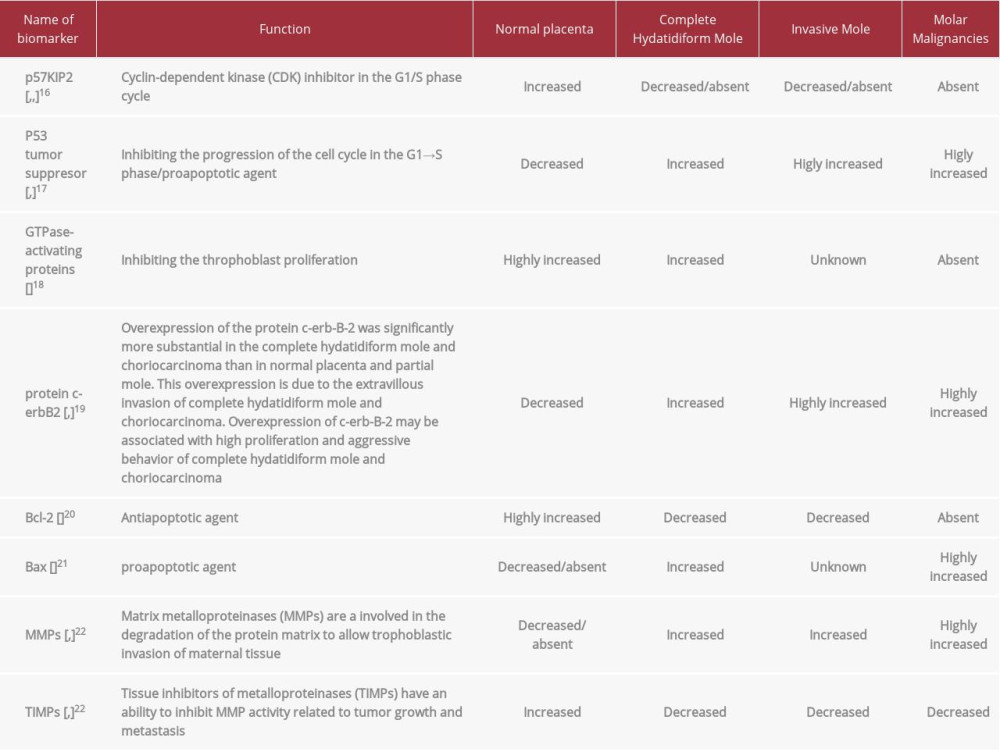 Table 2.. Biomarkers that have been suggested to plays a role in the transformation from hydatidiform mole to invasive mole.
Table 2.. Biomarkers that have been suggested to plays a role in the transformation from hydatidiform mole to invasive mole. Table 1.. Reported cases of invasive mole rapid transformation after curettage procedure.
Table 1.. Reported cases of invasive mole rapid transformation after curettage procedure. Table 2.. Biomarkers that have been suggested to plays a role in the transformation from hydatidiform mole to invasive mole.
Table 2.. Biomarkers that have been suggested to plays a role in the transformation from hydatidiform mole to invasive mole. In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








