21 October 2022: Articles 
Brain Abscesses Caused by in a 44-Year Old Woman with Multiple Myeloma: A Rare Case and Review of the Literature
Challenging differential diagnosis, Diagnostic / therapeutic accidents, Management of emergency care, Rare disease, Rare coexistence of disease or pathology
Khaled Sayer1ABCDEFG, Yousef A.I. AbouseduDOI: 10.12659/AJCR.937952
Am J Case Rep 2022; 23:e937952
Abstract
BACKGROUND: Central nervous system infection by the Nocardia species is associated with high morbidity and mortality. Its occurrence in patients with multiple myeloma is rare and acquisition of the infection in such patients was associated with the use of novel therapeutic agents (eg, bortezomib and lenalidomide) or bone marrow transplantation. Here, we report the first case of Nocardia brain abscesses in a patient with multiple myeloma, without the above risk factors.
CASE REPORT: A 44-year-old woman with IgG-kappa type multiple myeloma presented with generalized tonic-clonic seizures. Magnetic resonance imaging of the brain revealed 3 space-occupying lesions in left frontal, left parietal, and right parietal regions. Craniotomy and enucleation of the left frontal lesion revealed an abscess. The culture result was Nocardia farcinica. The patient was treated with meropenem, amikacin, and trimethoprim-sulfamethoxazole for 6 weeks, followed by trimethoprim-sulfamethoxazole for 12 months, with good outcome.
CONCLUSIONS: Cerebral nocardiosis is a rare entity and its occurrence in our case may hint toward myeloma-associated humoral immune dysfunction as a pathogenesis and the importance of humoral immunity in the defense against this infection. However, chemotherapy-induced cell-mediated dysfunction cannot be ruled out as a risk factor for the infection. Despite its rarity, this case aims to raise awareness of the condition and reiterate the importance of considering the rare but life-threatening conditions in the differential diagnosis of brain lesions, especially when there is a misdiagnosis of the radiological findings, as occurred in this and previous cases; this avoids delays in appropriate surgical and medical treatment, which can affect outcomes.
Keywords: brain abscess, Central Nervous System Bacterial Infections, Central Nervous System Infections, Multiple Myeloma, Nocardia, Nocardia farcinica, Female, Humans, Adult, Trimethoprim, Sulfamethoxazole Drug Combination, meropenem, amikacin, Bortezomib, lenalidomide, Nocardia Infections, Antineoplastic Agents, Immunoglobulin G
Background
A
A
Case Report
A 44-year-old woman was transferred to our Neurosurgical Unit from the Intensive Care Unit of Kuwait Cancer Control Center. Her past medical history was significant for diabetes, hyper-tension, and hysterectomy 6 years earlier, which was due to dysfunctional uterine bleeding. A year prior to her current presentation, she reported having persistent back pain, and further assessment revealed a stable L3 fracture. On further investigation, multiple myeloma (IgG kappa type; International Staging System-IIa) was diagnosed 8 months prior to her current presentation. The patient was started on cyclophosphamide, thalidomide, and dexamethasone and zoledronic acid. Her condition improved after the third cycle, and she declined to undergo bone marrow transplant assessment. She was not on any prophylactic antibiotics. Two months earlier, she developed a subcutaneous abscess on the left thigh, for which she underwent incision and drainage. Results of the cultures revealed mixed bacterial growth without isolating a specific pathogenic strain. Five days prior to transfer, the patient presented with generalized tonic-clonic seizures, which were difficult to control. As a result, she was sedated, intubated, and ventilated. Computed tomography (CT) and magnetic resonance imaging (MRI) of the brain revealed 3 space-occupying lesions: multi-compartment left deep frontal (4.6×3.8×3.2 cm), left parietal (1.2×2.2×3 cm), and right parietal (1.6×1.3×1.5 cm) (Figure 1). These lesions were cystic, with clear margins, and their walls were contrast-enhancing and surrounded by extensive edema, causing significant mass effect and midline shift. Cervical spine MRI and chest X-ray were normal. Thoracic and lumar spine MRI were only significant for several lytic lesions and fractures involving the thoraco-lumbar vertebrae, due to multiple myeloma.
Despite normal inflammatory markers and the fact that the radiological findings were interpreted as multiple brain metastasis, the patient still received empirical treatment with piper-acillin-tazobactam and vancomycin as a precaution in case these lesions were found to be abscesses during surgery. At the time of assessment, the patient’s body temperature was 37.5°C, heart rate was 58 beats/min, and blood pressure was 110/70 mmHg. The patient was sedated with remifentanyl, intubated, and ventilated. Despite the sedation, she was opening her eyes spontaneously and obeying commands (Glasgow Coma Scale score was E4, VIntubated, M6). On further examination, the patient was paraplegic (Medical Research Council [MRC] power scale 0/5 in both lower limbs) and had severe weakness in the upper limbs (MRC power scale 2/5 and 1/5 in the right upper limb and left upper limb, respectively). No sensory level was noted. Deep tendon reflexes were sluggish throughout.
The patient underwent left frontal craniotomy with total excision of the left frontal space-occupying lesions. Intraoperative findings were conclusvie for the diagnosis of an abscess, and samples were sent for microbiology and histopathological assessment. Micropscopy and staining revealed slender, branching, gram-positive bacilli, which were weakly acid-fast and morphologically resembling
The left and right posterior fronto-parietal lesions were not dealt with because they were small and in eloquent areas. The patient was extubated the next day, and the postoperative non-contrast CT scan of the brain showed the left frontal lesions appearing to be no longer visible (Figure 2). She remained at our neurosurgical institute for around 3 weeks before being transferred to the referring hospital to continue medical treatment. Prior to being transferred, the Glasgow Coma Scale score was 15/15 and power in all limbs showed discrete improvement.
The antibiotic regimen was changed to intravenous meropenem, amikacin, and trimethoprim-sulfamethoxazole for 6 weeks, followed by trimethoprim-sulfamethoxazole for 12 months, with very good neurological status and recovery. To the best of our knowledge, no additonal abscess formation occurred.
Discussion
To date, this is the seventh reported case of
It is estimated that about 86% of all
The optimal management approach for cerebral nocardiosis has not yet been established. However, craniotomy and enucleation of the abscess followed by prolonged antimicrobial therapy based on sensitivity results has been the preferred treatment option and is associated with a lower relapse of infection and reduced mortality of 24% [17,20,27,28,32,33]. In contrast, mortality rates with antimicrobial therapy alone and aspiration alone were 30% and 50%, respectively [28].
The prognosis of cerebral nocardiosis carries a high mortality and morbidity among all brain abscesses [17,27,34]. A significantly higher mortality rate of 66% has been found in multiple
Conclusions
Recognition of cerebral nocardiosis requires a high index of suspicion and early aggressive treatment with surgical enucleation of the abscess wall, prolonged antibiotics, and long-term surveillance, which are essential to prevent infection relapse, morbidity, and mortality.
Figures
References:
1.. Tamarit M, Poveda P, Barón M, Del Pozo JM, Four cases of nocardial brain abscess: Surg Neurol Int, 2012; 3; 88
2.. Corti ME, Villafañe-Fioti MF, Nocardiosis: A review: Int J Infect Dis, 2003; 7(4); 243-50
3.. Monticelli J, Luzzati R, Maurel C: Mediterr J Hematol Infect Dis, 2015; 7(1); e2015011
4.. Pamukçuoğlu M, Emmez H, Tunçcan OG: Hematology, 2014; 19(3); 158-62
5.. Chouciño C, Goodman SA, Greer JP, Nocardial infections in bone marrow transplant recipients: Clin Infect Dis, 1996; 23(5); 1012-19
6.. AlSamman S, Hayner C, Blatt S: Chest, 2014; 146(4); 165A
7.. Xu N, Li L, Lei W, Qian W: Brain Sci, 2021; 11(9); 1204
8.. Matin A, Sharma S, Mathur P, Apewokin SK, Myelosuppression-sparing treatment of central nervous system nocardiosis in a multiple myeloma patient utilizing a tedizolid-based regimen: A case report: Int J Antimicrob Agents, 2017; 49(4); 488-92
9.. Lerner PI, Nocardiosis: Clin Infect Dis, 1996; 22(6); 891-905
10.. Rawat D, Rajasurya V, Chakraborty RK, Sharma S, Nocardiosis: Stat Pearls August 3, 2021, Treasure Island (FL), StatPearls Publishing
11.. Wilson JW, Nocardiosis: Updates and clinical overview: Mayo Clin Proc, 2012; 87(4); 403-7
12.. Barnaud G, Deschamps C, Manceron V: J Clin Microbiol, 2005; 43(9); 4895-97
13.. Kilincer C, Hamamcioglu MK, Simsek O, Nocardial brain abscess: Review of clinical management: J Clin Neurosci, 2006; 13(4); 481-85
14.. McNeil MM, Brown JM, The medically important aerobic actinomycetes: Epidemiology and microbiology: Clin Microbiol Rev, 1994; 7(3); 357-417
15.. Menkü A, Kurtsoy A, Tucer B, Nocardia brain abscess mimicking brain tumour in immunocompetent patients: Report of two cases and review of the literature: Acta Neurochir (Wien), 2004; 146(4); 411-14
16.. Fleetwood IG, Embil JM, Ross IB: Surg Neurol, 2000; 53(6); 605-10
17.. Patil A, Cherian A, Iype T, Sandeep P, Nocardial brain abscess in an immunocompetent individual: Neurol India, 2011; 59(5); 779-82
18.. Iannotti CA, Hall GS, Procop GW: Surg Neurol, 2009; 72(1); 74-79
19.. Dominguez DC, Antony SJ, Actinomyces and nocardia infections in immunocompromised and nonimmunocompromised patients: J Natl Med Assoc, 1999; 91(1); 35-39
20.. Dias M, Nagarathna S, Mahadevan A, Nocardial brain abscess in an immunocompetent host: Indian J Med Microbiol, 2008; 26(3); 274-77
21.. König C, Kleber M, Reinhardt H, Incidence, risk factors, and implemented prophylaxis of varicella zoster virus infection, including complicated varicella zoster virus and herpes simplex virus infections, in lenalidomide-treated multiple myeloma patients: Ann Hematol, 2014; 93(3); 479-84
22.. Teo SK, Properties of thalidomide and its analogues: Implications for anti-cancer therapy: AAPS J, 2005; 7(1); E14-19
23.. Murray KJ, Ackerman SK, Chou SN, Douglas SD: Neurosurgery, 1977; 1(3); 297-299
24.. Kalambokis GN, Christou L, Tsianos EV, Multiple myeloma presenting with an acute bacterial infection: Int J Lab Hematol, 2009; 31(4); 375-83
25.. Beaman BL, Beaman L: Clin Microbiol Rev, 1994; 7(2); 213-64
26.. Anagnostou T, Arvanitis M, Kourkoumpetis TK, Nocardiosis of the central nervous system: Experience from a general hospital and review of 84 cases from the literature: Medicine (Baltimore), 2014; 93(1); 19-32
27.. Kennedy KJ, Chung KH, Bowden FJ, A cluster of nocardial brain abscesses: Surg Neurol, 2007; 68(1); 43-49
28.. Mamelak AN, Obana WG, Flaherty JF, Rosenblum ML, Nocardial brain abscess: Treatment strategies and factors influencing outcome: Neurosurgery, 1994; 35(4); 622-31
29.. Kim J, Minamoto GY, Grieco MH, Nocardial infection as a complication of AIDS: Report of six cases and review: Rev Infect Dis, 1991; 13(4); 624-29
30.. Beaman BL, Boiron P, Beaman L: J Med Vet Mycol, 1992; 30(Suppl. 1); 317-31
31.. Sorerell TC, Mitchell DH, Iredell JR, Chen SC: Mandell, Douglas, and Bennett’s Principles and practice of infectious disease, 2010; 3199-207, Philadelphia, Churchill Livingstone
32.. Lin YJ, Yang KY, Ho JT, Nocardial brain abscess: J Clin Neurosci, 2010; 17(2); 250-53
33.. Valarezo J, Cohen JE, Valarezo L, Nocardial cerebral abscess: Report of three cases and review of the current neurosurgical management: Neurol Res, 2003; 25(1); 27-30
34.. Zakaria A, Elwatidy S, Elgamal E: Acta Neurochir (Wien), 2008; 150(10); 1097-101
35.. Lee GY, Daniel RT, Brophy BP, Reilly PL, Surgical treatment of nocardial brain abscesses: Neurosurgery, 2002; 51(3); 668-72
Figures
Tables
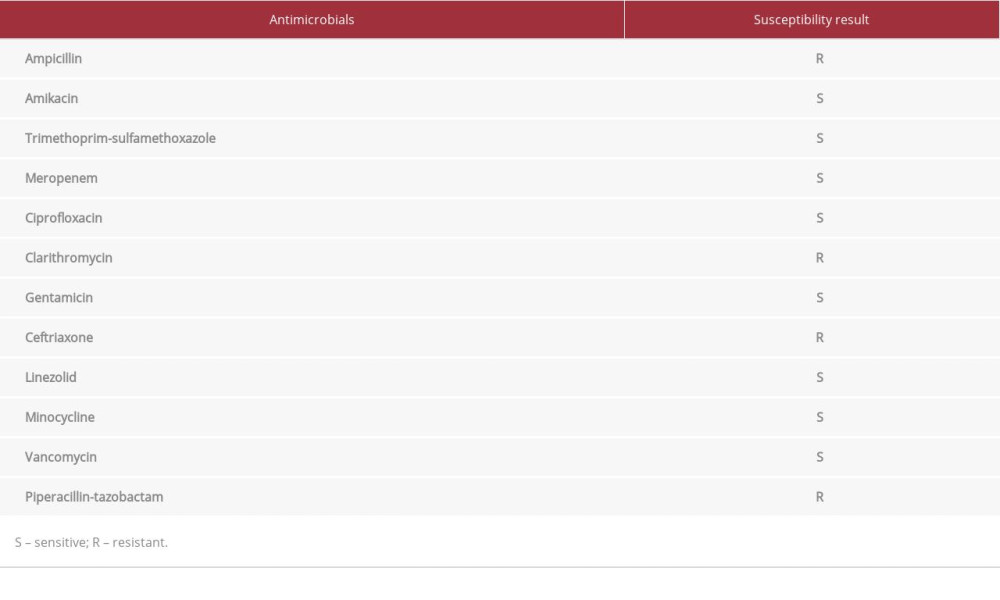 Table 1.. Susceptibility of Nocardia farcinica isolate to different antimicrobials.
Table 1.. Susceptibility of Nocardia farcinica isolate to different antimicrobials.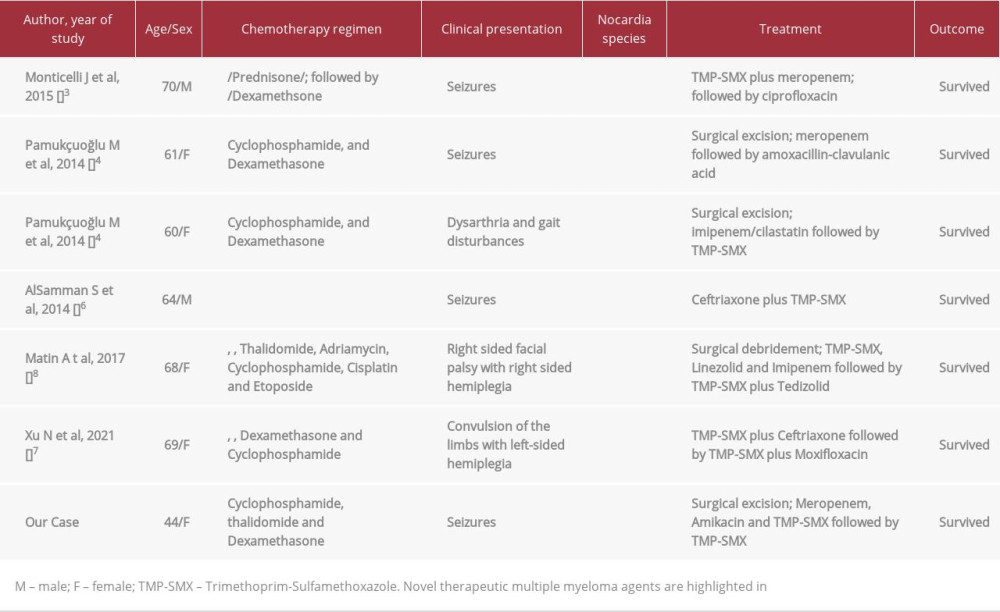 Table 2.. Summary of previously published case reports of cerebral nocardiosis in patients with multiple myeloma.
Table 2.. Summary of previously published case reports of cerebral nocardiosis in patients with multiple myeloma. Table 1.. Susceptibility of Nocardia farcinica isolate to different antimicrobials.
Table 1.. Susceptibility of Nocardia farcinica isolate to different antimicrobials. Table 2.. Summary of previously published case reports of cerebral nocardiosis in patients with multiple myeloma.
Table 2.. Summary of previously published case reports of cerebral nocardiosis in patients with multiple myeloma. In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








