04 April 2023: Articles 
Recurrent Reversible Stroke-Like Encephalopathy After 5-Fluorouracil (5-FU) Chemotherapy: A Case Report and Literature Review
Unusual clinical course, Mistake in diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Unexpected drug reaction, Clinical situation which can not be reproduced for ethical reasons
Kurniawan Agung Yuwono1ABCDEF, Susanna Hilda HutajuluDOI: 10.12659/AJCR.938437
Am J Case Rep 2023; 24:e938437
Abstract
BACKGROUND: Chemotherapy based on 5-fluorouracil (5-FU) is a well-established treatment for solid cancers, including metastatic or advanced colon cancer. Despite its efficacy, 5-FU can cause rare but serious adverse events such as acute neurotoxicity, which presents as symptoms similar to stroke.
CASE REPORT: We report the case of a patient who was diagnosed with stage IV colorectal cancer and who underwent chemotherapy with a high dose of 5-FU as part of the FOLFIRI (Folinic Acid, Fluorouracil, Irinotecan) treatment plan. During the seventh, eighth, and ninth cycles of chemotherapy, the patient suffered from severe encephalopathy, and the cause of this condition was determined to the 46-hour continuous intravenous infusion of 5-FU, which was part of the FOLFIRI regimen.
CONCLUSIONS: 5-FU-induced hyperammonemic encephalopathy is a rare but serious adverse event that requires immediate recognition and treatment. The first step in managing this condition is to halt the 5-FU infusion and provide the patient with high volumes of fluid. Although most cases of 5-FU-induced encephalopathy resolve spontaneously, recurrence is possible if the drug is re-administered to the same patient. Therefore, it is crucial for healthcare providers to closely monitor patients receiving 5-FU chemotherapy and be aware of the signs and symptoms of hyperammonemic encephalopathy. Early intervention can prevent further complications and ensure the best possible outcome for the patient. It is important to note that while 5-FU-induced hyperammonemic encephalopathy is rare, it highlights the importance of closely monitoring patients receiving chemotherapy to identify and treat adverse events promptly. This can help improve patient outcomes and prevent serious long-term complications.
Keywords: Colorectal Neoplasms, IFL Protocol, ischemic stroke, Humans, Fluorouracil, Antineoplastic Combined Chemotherapy Protocols, irinotecan, Stroke, cerebral infarction, Infusions, Intravenous
Background
5-Fluorouracil (5-FU)-based chemotherapy has been used in various solid tumors, including metastatic or advanced colon cancer [1]. The common adverse effects of 5-FU-based chemo-therapy include diarrhea, mucositis, and myelosuppression [2]. Acute stroke-like neurotoxicity after 5-FU chemotherapy is a rare adverse effect. We present the case of a man with stage IV colorectal cancer who received FOLFIRI regimen chemotherapy with a high dose of 5-FU (2500 mg/m2 continuous infusion for 46 hours every 2 weeks). During the seventh, eighth, and ninth cycles of chemotherapy, the patient experienced acute encephalopathy, which manifested as a loss of consciousness and right hemiparesis. Encephalopathy symptoms began shortly after the 46-hour continuous intravenous 5-FU infusion and resolved within 24 hours.
Case Report
A 49-year-old man with no known history or risk factors for cerebrovascular disease was diagnosed with stage IV sigmoid adenocarcinoma (Figure 1) with liver metastases (Figure 2). After undergoing exploratory laparotomy, bowel resection, and stoma placement, the patient received adjuvant chemotherapy with the Xelox regimen, which included Xeloda and oxaliplatin 3 times a week for 8 cycles. During the fifth cycle of the Xelox regimen, the patient’s renal function deteriorated (acute kidney injury grade III), and he required emergency hemodialysis due to encephalopathy uremicum. Ultrasonography of the kidneys revealed no indication of hydronephrosis, and the border between the renal cortex and medulla was unclear. The Xelox regimen was thereafter discontinued.
We discovered a residual tumor in the rectum, multiple liver metastases, and no metastases in the cerebral or thoracic cavities after re-staging with multislice computed tomography (MSCT) brain, thorax, and abdominal, following the 5th cycle of Xelox discontinuance. The regimen was switched to a second-line regimen consisting of FOLFIRI administered every 3 weeks for 12 cycles.
On the seventh cycle of FOLFIRI, the patient became unconscious during the final continuous infusion of 5-FU (2400 mg/m2 for 46 hours). A neurological examination revealed that he had an isochoric pupil, a normal light pupillary response, weakness in the right limbs, and no meningeal sign or pathological reflex. An MSCT brain without contrast revealed a subacute infarction in the left capsula externa and an old infarction in the cerebellar dextra (Figure 3). Based on clinical and MSCT brain findings, the patient was subsequently diagnosed with an acute ischemic stroke, and antiplatelet treatment was administered. After 24 hours of commencement, he regained full consciousness, and the paralysis in his right extremities completely resolved.
The patient was discharged from the hospital with mild nausea after completing the eighth FOLFIRI regimen cycle. However, a few hours after discharge from the hospital, his family stated that he had gradually become unconscious and developed right hemiparesis, presenting symptoms consistent with those observed after the seventh cycle of chemotherapy. The next day, the symptoms virtually disappeared. The patient’s family decided not to take him to the hospital.
During the ninth round of chemotherapy, near the end of a 46-hour 5-FU infusion, the patient became unconscious, with a Glasgow Coma Scale (GCS) score of E2M4V2 and weakness in the right extremities. Vital signs were normal (blood pressure of 137/87 mmHg, heart rate of 94 beats per minute, breath rate of 18 times per minute, the temperature of 36.5ºC, and peripheral oxygen saturation of 98% on room air), with no electrolyte or glucose level abnormalities, and a normal sinus rhythm ECG. The patient was diagnosed with delirium and was found to have an isochoric pupil with a normal light reaction, weakness in the right extremities, no meningeal sign, and no pathological reflex. There is an increase in blood ammonia (143.8 mg/dL) (Table 1). We assessed the patient with 5FU-induced encephalopathy and administered rehydration and laxatives, resulting in recovery of consciousness and right hemiparesis. Following 2 days of observation, the patient was discharged home.
A week after being discharged from an outpatient cancer clinic, the patient was completely conscious and capable of performing activities of daily living (ADL) with minimal help. We evaluated ammonia and MSCT brain contrast using contrast.
The ammonia level rose (152.6 ug/dL), and the MSCT brain with contrast was within the normal range (no infarction and no metastasis). The patient planned to change the regimen from 5-FU to Capecitabine. The patient’s clinical course during FOLFIRI chemotherapy and the neurological deficit is described in Figure 4.
The patient’s cognition baseline is fully alert, and he can perform daily life activities with minimum help. According to the patient, he never drank alcohol because it is forbidden based on the patient’s Muslim beliefs. The patient’s psychological health revealed no evidence of significant depression or hallucinations. The medications taken by the patient to treat nausea were ondansetron and dexamethasone, which have no significant impact on decreased consciousness.
Naranjo’s probability score for adverse drug reactions (ADRs) as shown in Table 2 was 10 (range 0-13), indicating a substantial association between 5-FU administration and encephalopathy.
Discussion
We reviewed the literature to identify studies describing the association between 5-FU use and encephalopathy.
Yeh and Cheng [3] described the clinical criteria for 5-FU-related encephalopathy as follows: (1) development of encephalopathy during or shortly after completion of 5-FU administration; (2) exclusion of other metabolic factors that may affect consciousness and mental functioning, such as hypoglycemia, organ failure, electrolyte imbalance, sepsis, and cancerous involvement of the central nervous system; and (3) exclusion of any adverse effects by concomitant medications.
The clinical signs of 5-FU-related encephalopathy include consciousness impairment, disorientation, lethargy, convulsions, and focal deficit [4]. Most patients with 5-FU-induced encephalopathy reported hyperammonemia, lactic acidosis, hyperammonemia-induced hypocapnia, and elevated liver transaminase [5]. Brain imaging can assist in ruling out other possible diagnoses, such as brain metastasis or stroke [6]. Electroencephalography may reveal diffuse slow or theta waves, indicative of metabolic encephalopathy [3].
Previous studies found that onset encephalopathy occurred most frequently after 2 to 3 days of chemotherapy, after 2 cycles of chemotherapy, and had increased blood ammonia levels (Table 3). The onset of encephalopathy in our patient occurred on day 2 of the seventh cycle of FOLFIRI chemotherapy and he had an increased blood ammonia level.
The precise etiology and pathogenesis of 5-FU-induced hyper-ammonemic encephalopathy have not been clearly explained. Fluorocitrate, the intermediate product of 5-FU metabolism, inhibits the Krebs tricarboxylic acid cycle, which can result in hyperammonemia by impairing the adenosine triphosphate-dependent urea cycle [7]. This hypothesis asserts that an accumulation of ammonia, a byproduct of 5-FU metabolism, results from administering 5-FU in high doses.
Dihydropyrimidine dehydrogenase (DPD) catalyzes the initial catabolic step in the 5FU breakdown pathway, converting 80% of 5FU to its inactive metabolite [8]. Patients who received an intermediate dose of 5-FU had DPD levels that were twice the normal range as compared to before therapy, resulting in a brief stagnation of 5-FU’s catabolites, which play a key role in the development of encephalopathy [9]. In addition, glutamine, the primary metabolic product of the ammonia metabolism in the brain, may also play an important role. The osmotic effect of accumulated intracellular glutamine can cause raised intracranial pressure syndrome and cerebral edema, as shown in many cases of hyperammonemic encephalopathy [6,7]. Severalfactors can contribute to the clinical manifestations of decreased consciousness observed in this patient. The first is a renal impairment with an estimated GFR of 45mL/min/1.73 m2. The second is dehydration, which results from nausea and vomiting as an adverse effect of chemotherapy. In addition, liver metastases can impair the liver’s capacity to catabolize the medication drug’s active substance.
During the patient’s Xelox-based first-line chemotherapy session, his liver function was normal. The decreased consciousness only occurred in the fifth series of chemotherapy due to increased urea levels. After cessation of treatment, renal function remained normal even after administration of a second-line chemotherapy regimen with FOLFIRI. Although the patient had liver metastases, the enzyme transaminases were only marginally elevated, indicating minor liver cell injury. Thus, the clinical manifestation of decreased consciousness caused by FOLFIRI chemotherapy is less likely to been associated with the previous chemotherapy residual in the liver and more likely to have been caused by a non-hepatic route.
In this case report, the clinical manifestations of decreased consciousness improved after the patient received hydration; therefore, he was not transferred to the intensive care unit and did not receive additional therapy. However, in a state of decreased consciousness accompanied by hyperammonemia, patients can be given Lactulose, which lowers ammonia synthesis and absorption in the intestine. Sugar metabolism in the colon exerts a laxative effect by increasing intraluminal gas production and osmolality, which decreases transit time and intraluminal pH. Moreover, Lactulose stimulates ammonia uptake by colonic bacteria, which use the trapped ammonia as a nitrogen source for protein production. This process is aided by lowering intestinal pH, which promotes the conversion of ammonia (NH3) produced by gut bacteria to ammonium (NH4+). Lactulose also reduces ammonia generation in the intestines [10].
Conclusions
There is no effective treatment for 5-FU-induced hyperammonemic encephalopathy. When 5-FU-induced hyperammonemic encephalopathy is suspected, it is essential to immediately discontinue 5-FU infusion and rehydrate with high volumes of fluid. For differential diagnosis, laboratory testing and imaging examinations should be undertaken. Most patients improve without problems or persistent neurological damage after supportive care, including termination of 5-FU therapy, hydration, and Lactulose enema.
This case report is essential for guiding clinical practice to include hyperammonemia as a differential diagnosis for decreased consciousness in cancer patients receiving 5-FU-based chemotherapy regimens so that this condition is not misinterpreted as recurrent stroke.
Although most cases of 5-FU-induced encephalopathy improve spontaneously, recurrence is possible when the drug is administered to the same patient. Therefore, it is challenging to determine whether an alternative therapeutic approach should be utilized.
Figures
References:
1.. Vodenkova S, Buchler T, Cervena K, 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future: Pharmacol Ther, 2020; 206; 107447
2.. Jose N, Joel A, Selvakumar RJ, Diagnosis and management of 5-fluorouracil (5-FU)-induced acute leukoencephalopathy: Lessons learnt from a single-centre case series: J Egypt Natl Canc Inst, 2022; 34; 22
3.. Yeh KH, Cheng AL, High-dose 5-fluorouracil infusional therapy is associated with hyperammonaemia, lactic acidosis and encephalopathy: Br J Cancer, 1997; 75; 464-65
4.. Boilève A, Thomas L, Lillo-Le Louët A, 5-Fluorouracil-induced hyper-ammonaemic encephalopathy: A French national survey: Eur J Cancer, 2020; 129; 32-40
5.. Formica V, Leary A, Cunningham D, 5-Fluorouracil can cross brain-blood barrier and cause encephalopathy: should we expect the same from capecitabine? A case report on capecitabine-induced central neurotoxicity progressing to coma: Cancer Chemother Pharmacol, 2006; 58; 276-78
6.. Nott L, Price TJ, Pittman K, Hyperammonemia encephalopathy: An important cause of neurological deterioration following chemotherapy: Leuk Lymphoma, 2007; 48; 1702-11
7.. Koenig H, Patel A, Biochemical basis for fluorouracil neurotoxicity. The role of Krebs cycle inhibition by fluoroacetate: Arch Neurol, 1970; 23; 155-60
8.. Heggie GD, Sommadossi JP, Cross DS, Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile: Cancer Res, 1987; 47; 2203-6
9.. Kim Y-A, Chung HC, Choi HJ, Intermediate dose 5-fluorouracil-induced encephalopathy: Jpn J Clin Oncol, 2006; 36; 55-59
10.. Cichoż-Lach H, Michalak A, Current pathogenetic aspects of hepatic encephalopathy and noncirrhotic hyperammonemic encephalopathy: World J Gastroenterol, 2013; 19; 26-34
11.. Yi HJ, Hong KS, Moon N, Acute hyperammonemic encephalopathy after 5-fluorouracil based chemotherapy: Ann Surg Treat Res, 2016; 90; 179-82
12.. Nguyen MT, Stoianovici R, Brunetti L, Chemotherapy induced stroke mimic: 5-Fluorouracil encephalopathy fulfilling criteria for tissue plasminogen activator therapy: Am J Emerg Med, 2017; 35; 1389-90
13.. Mitani S, Kadowaki S, Komori A, Acute hyperammonemic encephalopathy after fluoropyrimidine-based chemotherapy: A case series and review of the literature: Medicine (Baltimore), 2017; 96; e6874
14.. Boilève A, Wicker C, Verret B, 5-Fluorouracil rechallenge after 5-fluorouracil-induced hyperammonemic encephalopathy: Anticancer Drugs, 2019; 30; 313-17
15.. Ihoriya H, Yamamoto H, Yamada T, Hyperammonemic encephalopathy in a patient receiving fluorouracil/oxaliplatin chemotherapy: Clin Case Rep, 2018; 6; 603-5
16.. Naik SS, Vanidassane I, Dhamija E, A unique presentation of 5-fluorouracil (5-FU) induced cerebral encephalopathy: Indian J Radiol Imaging, 2020; 30; 214-17
Figures
Tables
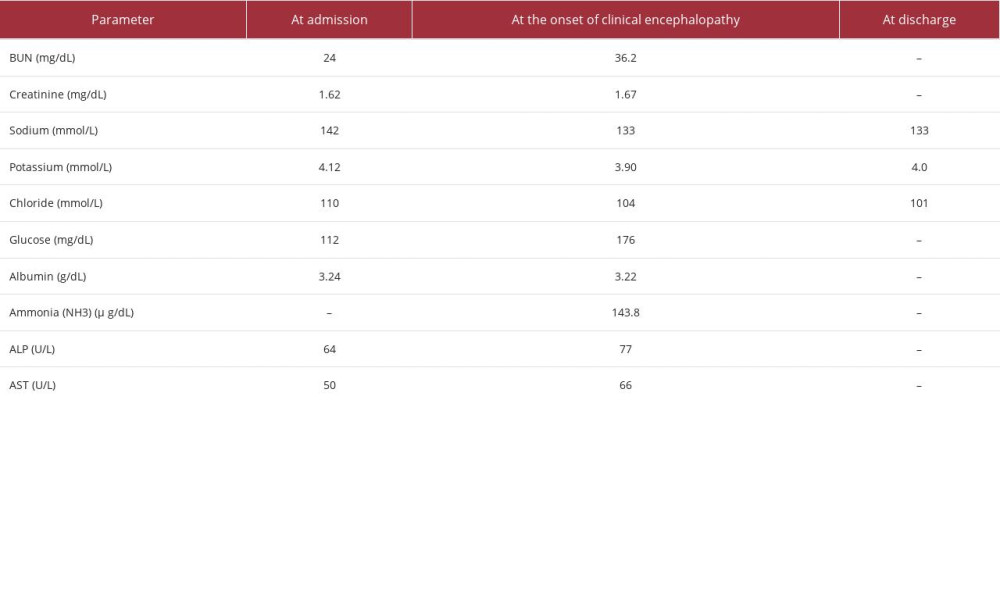 Table 1.. Laboratory parameters during the seventh cycle of chemotherapy.
Table 1.. Laboratory parameters during the seventh cycle of chemotherapy.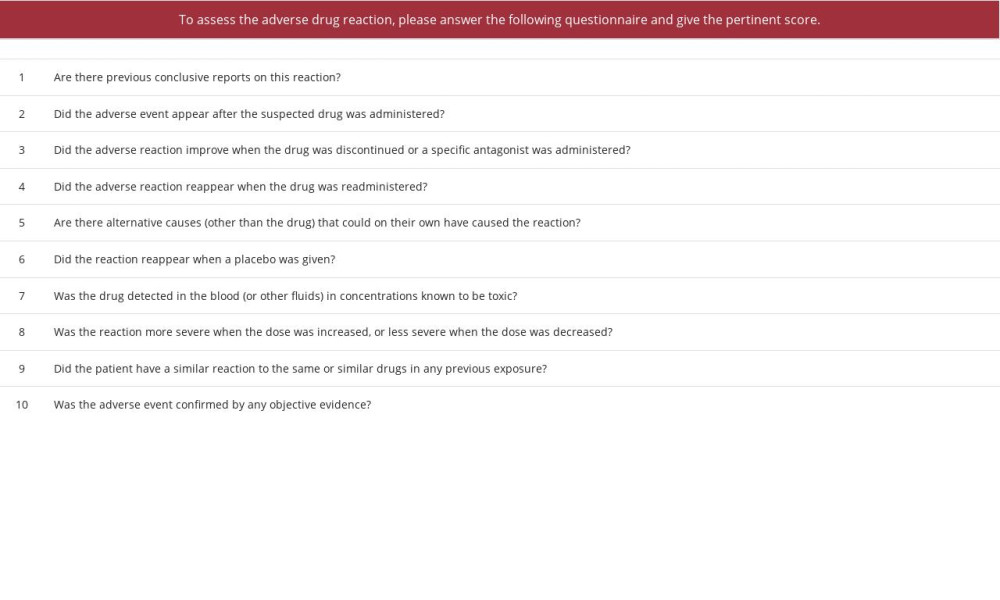 Table 2.. Adverse drug reactions (ADRs) probability scale.
Table 2.. Adverse drug reactions (ADRs) probability scale.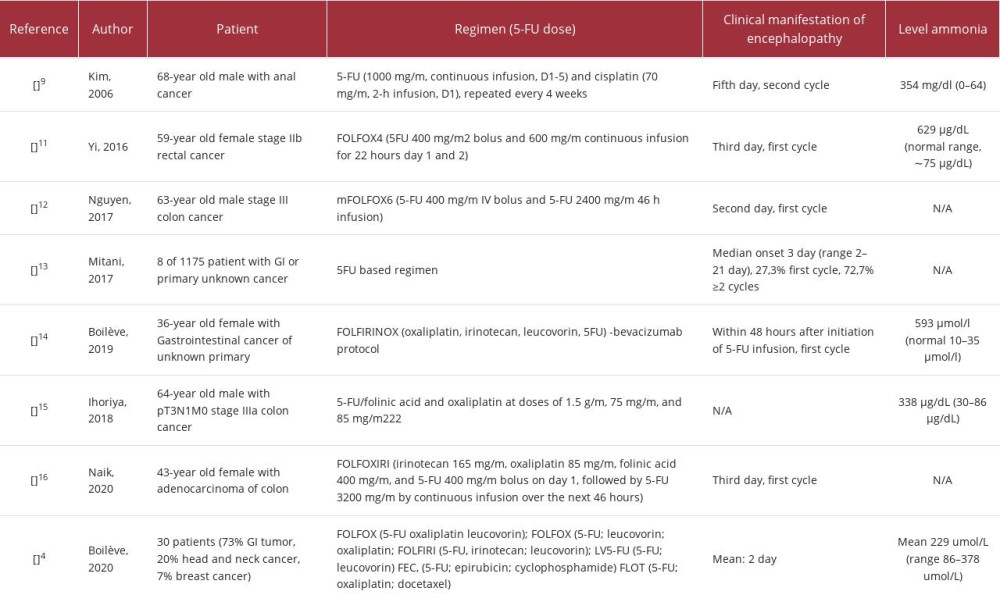 Table 3.. Cases of encephalopathy associated with the administration of 5-FU.
Table 3.. Cases of encephalopathy associated with the administration of 5-FU. Table 1.. Laboratory parameters during the seventh cycle of chemotherapy.
Table 1.. Laboratory parameters during the seventh cycle of chemotherapy. Table 2.. Adverse drug reactions (ADRs) probability scale.
Table 2.. Adverse drug reactions (ADRs) probability scale. Table 3.. Cases of encephalopathy associated with the administration of 5-FU.
Table 3.. Cases of encephalopathy associated with the administration of 5-FU. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








