15 July 2021: Articles 
Isolated Adrenocorticotropic Hormone Deficiency Associated with Severe Hyperkalemia During Pembrolizumab Therapy in a Patient with Ureteral Cancer and an Ileal Conduit: A Case Report and Literature Review
Unusual clinical course
Yudai Hinata1BCD, Nobumasa Ohara1ABCDEF*, Yuhki Sakurai1CD, Ryo Koda2BCD, Yuichiro Yoneoka3CD, Toshinori Takada4CD, Noboru Hara5BD, Tsutomu Nishiyama5BDDOI: 10.12659/AJCR.931639
Am J Case Rep 2021; 22:e931639
Abstract
BACKGROUND: Immune checkpoint inhibitors (ICIs) are anticancer medications that enhance the antitumor immune response. The clinical benefit afforded by ICIs, however, can be accompanied by immune-related adverse events (IRAEs). One of the common endocrine IRAEs is hypophysitis, which often causes hypopituitarism with secondary adrenal insufficiency (AI). Secondary AI, including isolated adrenocorticotropic hormone (ACTH) deficiency (IAD), is often associated with hyponatremia. Here, we report an unusual case of ICI-related IAD associated with severe hyperkalemia.
CASE REPORT: A 78-year-old woman who had an ileal conduit, chronic kidney disease, type 2 diabetes mellitus, and hypertension and was taking an angiotensin II receptor blocker began treatment for advanced ureteral cancer with the anti-programmed cell death protein 1 inhibitor pembrolizumab. The therapy effectively controlled the cancer, but 4 1/2 months after starting it, the patient developed anorexia, general weakness, and muscle pain and was diagnosed with IAD associated with severe hyperkalemia and hyperchloremic metabolic acidosis. She recovered after prompt administration of corticosteroids and treatment with sodium bicarbonate, glucose/insulin, and cation exchange resins.
CONCLUSIONS: Hyperkalemia is a common symptom of primary AI but is less common in patients with central AI because a lack of ACTH does not cause aldosterone deficiency and mineralocorticoid action is preserved. The present case demonstrates the need for physicians to be aware of severe hyperkalemia as a life-threatening complication of secondary AI induced by ICIs, particularly in patients with predisposing factors, such as kidney dysfunction, diabetes mellitus, an ileal conduit, and renin-angiotensin-aldosterone system inhibitor use.
Keywords: adrenal insufficiency, Diabetes Mellitus, Hydrocortisone, Hyperkalemia, Renal Insufficiency, Chronic, Urinary Diversion, Adrenocorticotropic Hormone, Antibodies, Monoclonal, Humanized, Diabetes Mellitus, Type 2, Ureteral Neoplasms
Background
Immune checkpoint inhibitors (ICIs) are anticancer medications that enhance the antitumor immune response by blocking negative regulators of T-cell function. The clinical benefit afforded by ICIs, however, can be accompanied by immune-related adverse events (IRAEs) that involve different organs, as well as the endocrine system [1]. One of the major endocrine IRAEs is hypophysitis, which often causes hypopituitarism associated with secondary adrenal insufficiency (AI) and can be fatal if not appropriately treated.
Hypophysitis is less frequent and exhibits a distinct clinical phenotype in patients treated with anti-programmed cell death protein 1 (PD-1) inhibitors compared with anti-cytotoxic T-lymphocyte antigen-4 inhibitors [2]. The time of onset of hypophysitis in patients on PD-1 inhibitors is longer, ranging from several months to years, and the symptoms are less severe. Patients do not usually present with headache or fever, and magnetic resonance imaging (MRI) of the sellar region usually does not reveal any abnormality, such as pituitary enlargement. Even in the absence of typical hypophysitis symptoms, however, PD-1 inhibitors often cause hypopituitarism with secondary AI [2,3]. In particular, many cases of isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) [4,5] have been reported [6–31]. Patients with anti-PD-1-related IAD present with cortisol deficiency symptoms, including anorexia, fatigue, and general weakness. In addition, hyponatremia is often a principal sign of anti-PD-1-induced IAD [8].
We report an unusual case of anti-PD-1-related IAD presenting with severe hyperkalemia. In addition, we review previously reported cases of IAD associated with PD-1 inhibitor therapy.
Case Report
A 78-year-old woman with advanced ureteral cancer who was on PD-1 inhibitor therapy with pembrolizumab was admitted to our hospital in November 2019 after experiencing anorexia, general weakness, and muscle pain for several days. The patient had a paternal family history of type 2 diabetes mellitus (T2D), hypertension, and cerebral infarction. She patient did not drink alcohol or smoke cigarettes. She was diagnosed with T2D, dyslipidemia, and hypertension associated with obesity at age 63 years and started medications, including the angiotensin II receptor blocker candesartan. She also had mild chronic kidney disease (CKD) associated with these metabolic disorders.
At the patient’s regular check-up in June 2018, a urine culture was positive for occult blood. Abdominal ultrasound, computed tomography (CT), and MRI revealed tumors in the bladder and the left lower part of the ureter. In August 2018, the patient underwent a laparoscopic left total nephroureterectomy, total cystectomy, and ileal conduit urinary diversion. Histopathological examination revealed invasive urothelial carcinoma in the bladder and left ureter. After surgery, the patient’s kidney function partially deteriorated, and her serum creatinine levels were 1.5 to 2.0 mg/dL. She also developed mild hyperkalemia and started treatment with dietary potassium restriction and oral calcium polystyrene sulfonate (30 g/d). In addition, she was presumed to have hyperchloremic metabolic acidosis (HMA) associated with her ileal conduit [32].
A CT scan performed in March 2019 detected metastasis in the left pelvis. The patient received 3 courses of chemotherapy with gemcitabine and carboplatin from April to June 2019 and then started maintenance chemotherapy with pembrolizumab (200 mg every 3 weeks) in early July 2019. Her morning plasma ACTH and cortisol levels just before starting pembrolizumab were normal (43.1 pg/mL and 12.5 μg/dL, respectively) (Table 1).
After 2 cycles of pembrolizumab therapy, in mid-August 2019, a blood test performed at noon showed a low plasma cortisol level of 5.4 μg/dL (ACTH, 19.4 pg/mL), indicating possible AI. A rapid ACTH stimulation test revealed a slightly low plasma cortisol level (17.1 μg/dL) (Table 2A) [33]. However, the patient presented with no symptoms suggestive of hypophysitis or AI, such as headache, fever, anorexia, or general weakness. A brain MRI detected no abnormality in the pituitary gland (Figure 1A, 1C). Therefore, she was closely followed with no corticosteroid replacement therapy.
In late August 2019, the patient’s morning plasma cortisol level returned to normal (12.2 μg/dL) (Table 1). She continued the pembrolizumab therapy, which effectively controlled her ureteral cancer and left pelvic metastasis. A CT performed in September 2019 revealed that the pelvic tumor was smaller and there were no new lesions. In November 2019, however, 5 days after the seventh cycle of pembrolizumab therapy, the patient developed anorexia, general weakness, back pain, and muscle pain in her extremities. In a few days, she had difficulty walking and was admitted to our hospital.
On admission, the patient’s body length, weight, temperature, blood pressure, and pulse rate, were 149.0 cm, 58.1 kg, 36.7°C, 99/54 mmHg, and 84 beats/min, respectively. Her mouth was slightly dry. No thyromegaly, heart murmurs, chest rales, abdominal pain, rash, skin pigmentation, or peripheral edema were detected. A blood gas analysis indicated metabolic acidemia (pH 7.204) associated with a low bicarbonate ion (HCO3) level (13.0 mmol/L) and a normal anion gap (9.8 mmol/L). Blood chemistry results revealed high levels of serum potassium (6.3 mEq/L) and chloride (113 mEq/L) but a low serum sodium level (134 mEq/L) (Table 3). In addition, the patient’s plasma cortisol level was low (1.4 μg/dL). An electrocardiogram revealed a normal sinus rhythm with no loss of P waves, widening of the QRS complex, or arrhythmia, but tall peak T waves were seen in the precordial leads.
Infusion therapy with normal saline to promote diuresis, sodium bicarbonate for HMA, and glucose/insulin were promptly administered to correct hyperkalemia (Figure 2). In addition, the patient began taking corticosteroid replacement therapy with oral hydrocortisone (15 mg/d) immediately after completing a rapid ACTH stimulation test, which indicated central AI (Table 2A). Her anorexia, general weakness, back pain, and muscle pain resolved within a few days and she became ambulatory. Infusion therapy was discontinued after the patient’s blood electrolytes (sodium, potassium, and chloride) and pH normalized. She subsequently was administered oral sodium bicarbonate (2 g/d) to treat her ileal conduit-related chronic HMA.
Dynamic tests for pituitary function revealed low ACTH and cortisol release after the administration of corticotropin-releasing hormone (CRH) (Table 2B, 2C); secretory reserves of thyroid-stimulating hormone, growth hormone, prolactin, follicle-stimulating hormone, and luteinizing hormone were normal for the patient’s age. These findings indicated IAD.
Brain MRI revealed no abnormality and excluded metastatic involvement of the pituitary gland, but not anti-PD-1-related hypophysitis (Figure 1B, 1D). Abdominal CT detected no abnormality (thus, no calcification or metastasis) in the adrenal glands.
Because the patient had not been exposed to other possible triggers of hypopituitarism with central AI, such as head trauma, pituitary surgery, or radiotherapy [34], and she had not received exogenous steroids during pembrolizumab therapy, she was diagnosed with IAD attributable to pembrolizumab-related hypophysitis.
The patient continued on oral hydrocortisone (15 mg/d), sodium bicarbonate (2 g/d), and calcium polystyrene sulfonate (30 g/d) and was discharged 18 days after admission. Her clinical course since then has been uneventful for more than 6 months.
Discussion
Table 4 summarizes the characteristics of the reported cases of anti-PD-1-related IAD [6–30]. Patients with different types of cancer developed IAD with symptoms of cortisol deficiency, such as anorexia, fatigue, and general weakness. The time between drug initiation and the development of IAD ranged from 1.5 to 15 months. Many patients presented with hyponatremia but their serum potassium levels were normal. In the present case, the patient developed IAD 4 1/2 months after initiation of a drug to treat advanced ureteral cancer and had severe hyperkalemia.
Hyperkalemia is a life-threatening electrolyte disturbance that causes muscle weakness, muscle pain, paralysis, and fatal cardiac arrhythmias [35]. Hyperkalemia is a common symptom of primary AI, which is associated with defective mineralocorticoid action due to cortisol and aldosterone deficiencies [33]. In contrast, hyperkalemia is less frequent in patients with central AI because a lack of ACTH does not cause an aldosterone deficiency and mineralocorticoid action is preserved [4,36]. To our knowledge, ours is the first reported case of anti-PD-1-related IAD presenting with severe hyperkalemia.
Potassium is a major intracellular cation. Hyperkalemia can result from disturbed cellular uptake of potassium, due to acidosis, impaired insulin action, or decreased b2 adrenergic activity, as well as disturbed renal excretion of potassium [35]. Disrupted potassium excretion can be due to kidney dysfunction or defective mineralocorticoid action. Certain medications, such as renin-angiotensin-aldosterone system (RAAS) inhibitors, also induce hyperkalemia, particularly in patients with kidney dysfunction. Although the precise mechanism remains unknown, ICIs cause hyperkalemia on rare occasions, regardless of endocrinopathies [37]. In the present case, cortisol deficiency together with ileal conduit-related HMA (loss of HCO3 and gain of chloride from the conduit) [32], CKD, T2D, and the use of a RAAS inhibitor probably led to the severe hyperkalemia. Prompt administration of corticosteroids and treatment with normal saline, sodium bicarbonate, glucose/insulin, and cation exchange resin, as well as discontinuation of the RAAS inhibitor, helped the patient recover from severe hyperkalemia (Figure 2).
Predisposing factors for pituitary IRAEs are not well known. However, studies have suggested associations between certain human leucocyte antigen haplotypes, such as HLA-DR15, and the development of ICI-related hypopituitarism with secondary AI [38]. Several laboratory abnormalities, such as eosinophilia [12] and hyponatremia [8], also can precede ICI-related secondary AI. Cases have been reported of ICI-related IAD developing after transient elevations in plasma ACTH and cortisol levels [39,40]. In addition, a patient with pembrolizumab-related IAD associated with gradually deteriorating pituitary and adrenal functions has been described; a CRH stimulation test performed 4 months before IAD onset showed a mild reduction in plasma ACTH and cortisol release [28]. In the present case, the patient exhibited transient reductions in plasma ACTH and cortisol levels 3 months before the development of IAD (Tables 1, 2A). Subsequently, her blood eosinophils and ACTH levels gradually increased. These findings suggest, in our case, that the transient reductions in ACTH and cortisol levels were probably related to anti-PD-1-related hypophysitis and the consequent IAD.
Conclusions
We have described a patient with pembrolizumab-related IAD who presented with severe hyperkalemia. The present case demonstrates the need for physicians to be aware of severe hyperkalemia as a life-threatening complication of secondary AI induced by ICIs, particularly in patients with predisposing factors, such as kidney dysfunction, T2DM, an ileal conduit, and RAAS inhibitor use.
Figures
Tables
Table 1.. Serial changes in levels of morning plasma adrenocorticotropic hormone and cortisol, serum electrolytes, and blood eosinophils, immediately before and during pembrolizumab therapy in 2019. All blood samples were collected in the morning.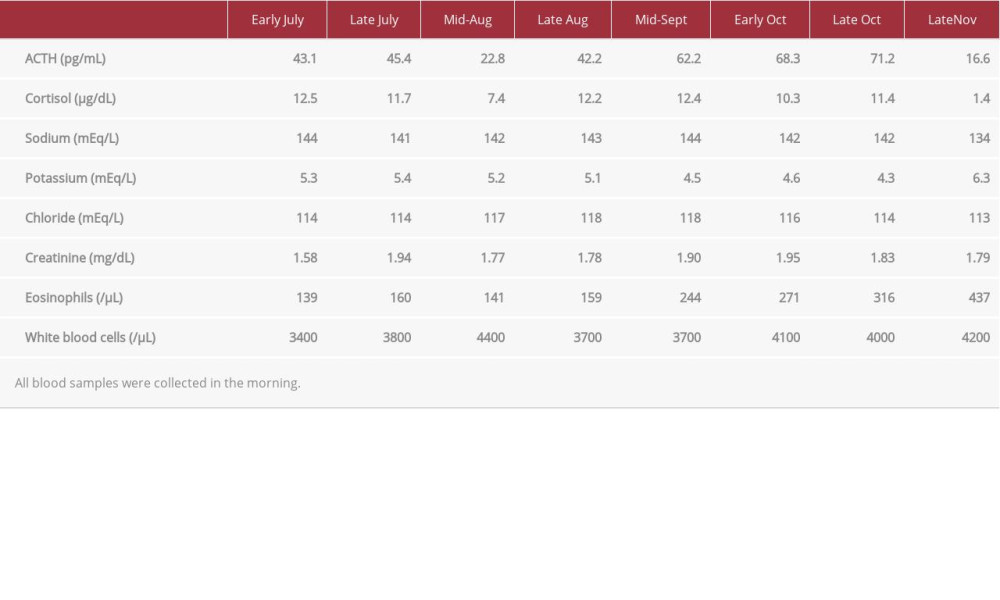 Table 2.. Endocrinological investigation.A: Rapid adrenocorticotropic hormone stimulation test.
Table 2.. Endocrinological investigation.A: Rapid adrenocorticotropic hormone stimulation test.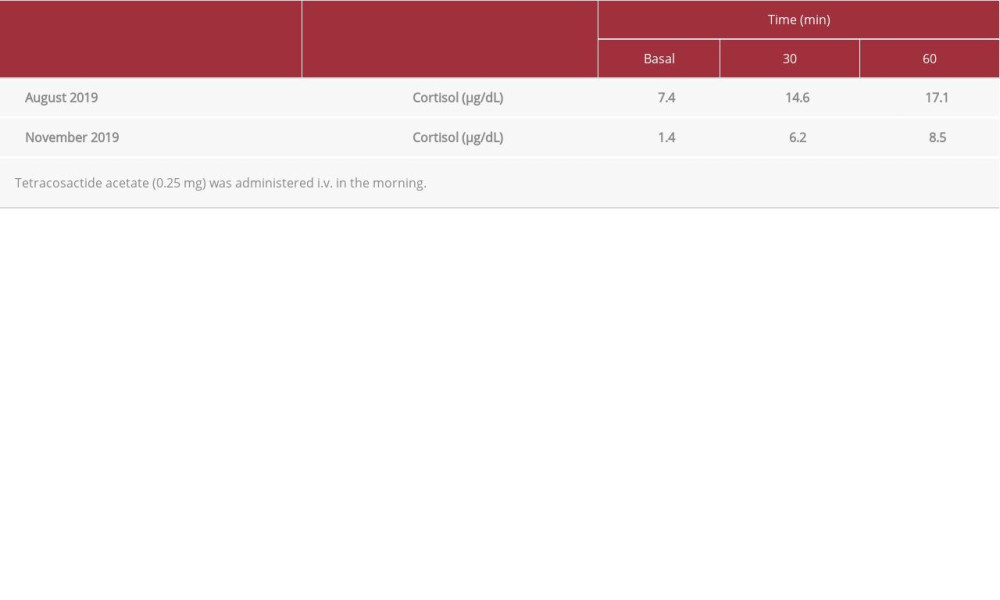 . B: Corticotropin-releasing hormone (CRH)/growth hormone-releasing factor (GRF)/thyrotropin-releasing hormone (TRH)/luteinizing hormone-releasing hormone (LHRH) stimulation test (November 2019).
. B: Corticotropin-releasing hormone (CRH)/growth hormone-releasing factor (GRF)/thyrotropin-releasing hormone (TRH)/luteinizing hormone-releasing hormone (LHRH) stimulation test (November 2019).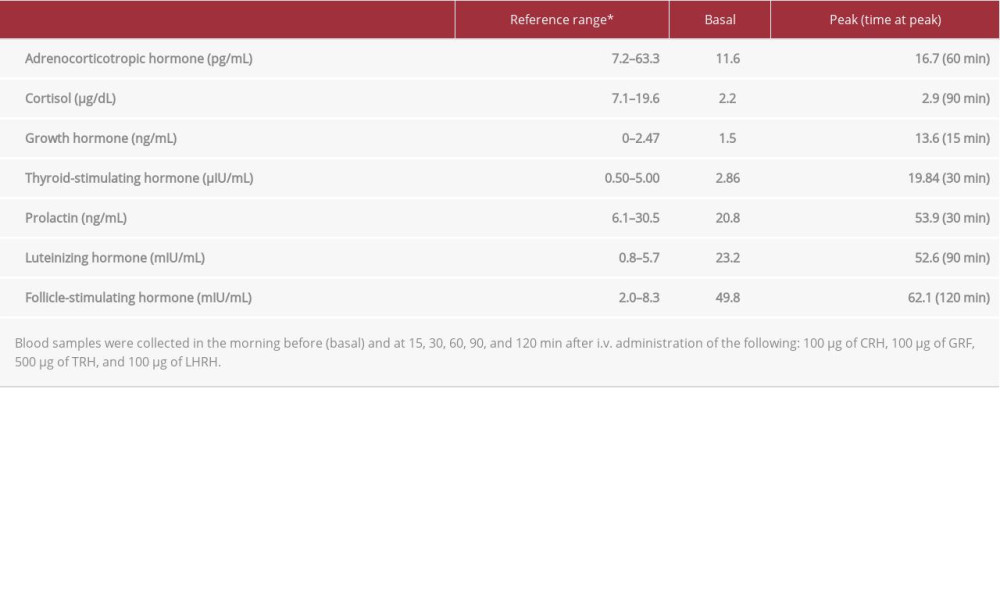 . C: Growth hormone-releasing peptide 2 (GHRP-2) stimulation test performed in November 2019.
. C: Growth hormone-releasing peptide 2 (GHRP-2) stimulation test performed in November 2019.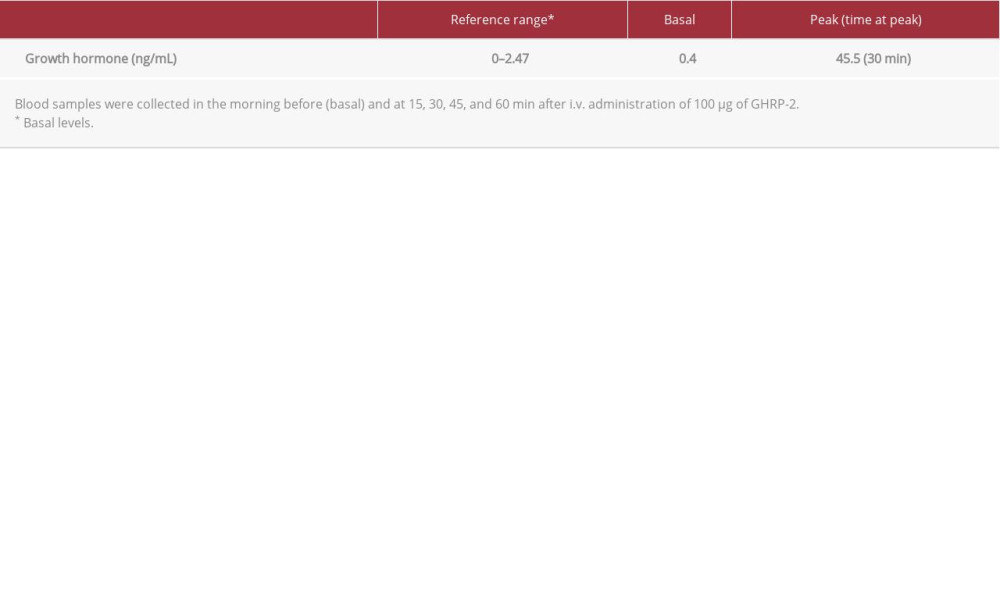 Table 3.. Laboratory findings on admission in November 2019.
Table 3.. Laboratory findings on admission in November 2019.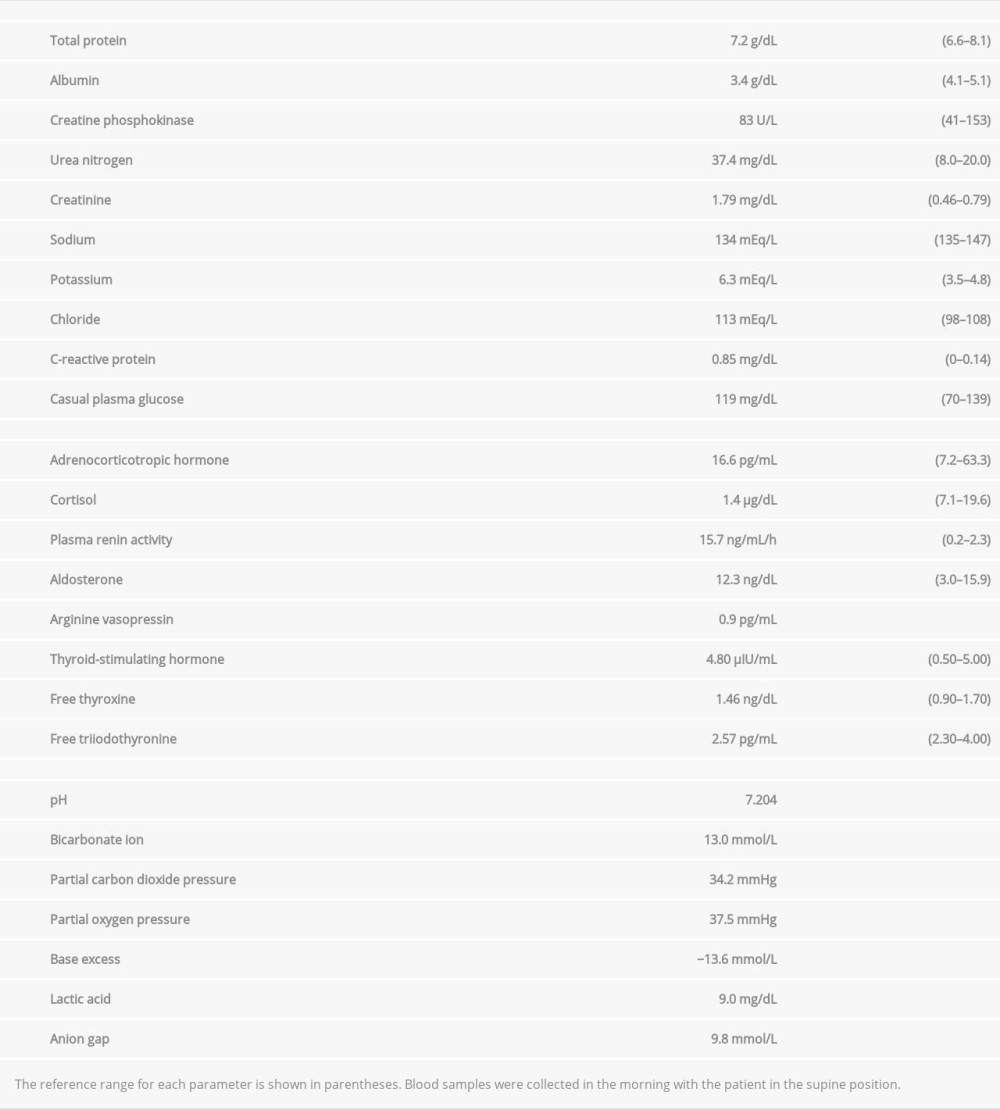 Table 4.. Summary of reports of patients who exhibited isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) associated with cancer treatment with programmed cell death 1 inhibitors.
Table 4.. Summary of reports of patients who exhibited isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) associated with cancer treatment with programmed cell death 1 inhibitors.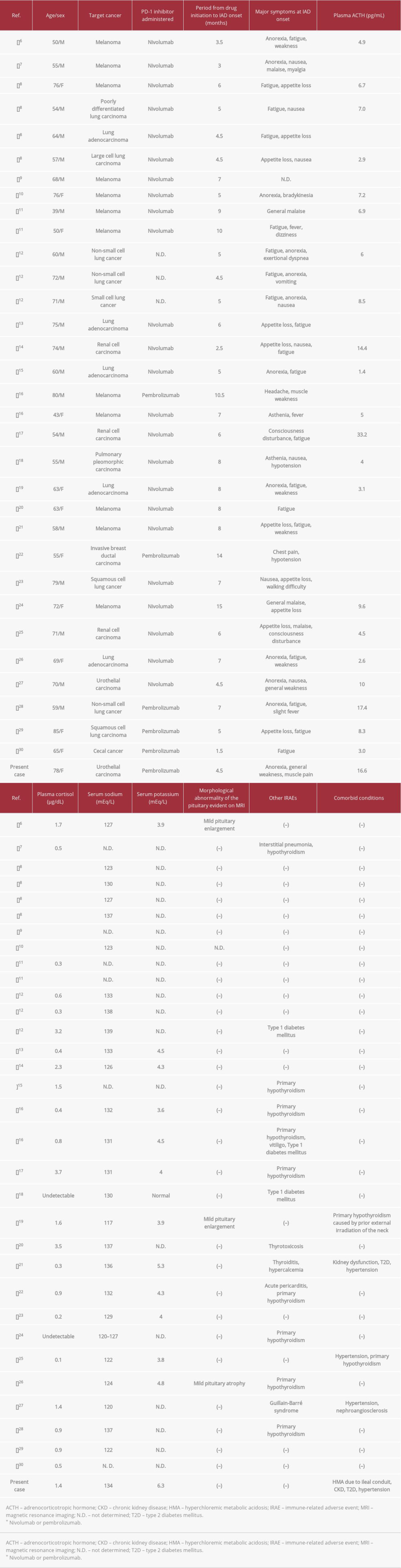
References:
1.. Sznol M, Postow MA, Davies MJ, Endocrine-related adverse events associated with immune checkpoint blockade and expert insights on their management: Cancer Treat Rev, 2017; 58; 70-76
2.. Faje A, Reynolds K, Zubiri L, Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis: Eur J Endocrinol, 2019; 181; 211-19
3.. Sonehara K, Tateishi K, Hirabayashi T, A case of lung adenocarcinoma with long-term response after late-onset pembrolizumab-induced acute adrenal insufficiency: Case Rep Oncol, 2021; 14; 1-7
4.. Andrioli M, Pecori Giraldi F, Cavagnini F, Isolated corticotrophin deficiency: Pituitary, 2006; 9; 289-95
5.. Percik R, Shlomai G, Tirosh A, Isolated autoimmune adrenocortico-tropic hormone deficiency: From a rare disease to the dominant cause of adrenal insufficiency related to check point inhibitors: Autoimmun Rev, 2020; 19; 102454
6.. Okano Y, Satoh T, Horiguchi K, Nivolumab-induced hypophysitis in a patient with advanced malignant melanoma: Endocr J, 2016; 63; 905-12
7.. Ishikawa M, Oashi K, Case of hypophysitis caused by nivolumab: J Dermatol, 2017; 44; 109-10
8.. Cho KY, Miyoshi H, Nakamura A, Hyponatremia can be a powerful predictor of the development of isolated ACTH deficiency associated with nivolumab treatment [Letter to the Editor]: Endocr J, 2017; 64; 235-36
9.. Fujimura T, Kambayashi Y, Furudate S, Isolated adrenocorticotropic hormone deficiency possibly caused by nivolumab in a metastatic melanoma patient: J Dermatol, 2017; 44; e13-14
10.. Narahira A, Yanagi T, Cho KY, Isolated adrenocorticotropic hormone deficiency associated with nivolumab therapy: J Dermatol, 2017; 44; e70
11.. Kitajima K, Ashida K, Wada N, Isolated ACTH deficiency probably induced by autoimmune-related mechanism evoked with nivolumab: Jpn J Clin Oncol, 2017; 18; 1-4
12.. Ariyasu R, Horiike A, Yoshizawa T, Adrenal insufficiency related to anti-programmed death-1 therapy: Anticancer Res, 2017; 37; 4229-32
13.. Takaya K, Sonoda M, Fuchigami A, Hiyoshi T, Isolated adrenocorticotropic hormone deficiency caused by nivolumab in a patient with metastatic lung cancer: Intern Med, 2017; 56; 2463-69
14.. Seki T, Yasuda A, Oki M, Secondary adrenal insufficiency following nivolumab therapy in a patient with metastatic renal cell carcinoma: Tokai J Exp Clin Med, 2017; 42; 115-20
15.. Kastrisiou M, Kostadima FL, Kefas A, Nivolumab-induced hypothyroidism and selective pituitary insufficiency in a patient with lung adenocarcinoma: A case report and review of the literature: ESMO Open, 2017; 2; e000217
16.. Lupi I, Brancatella A, Cosottini M, Clinical heterogeneity of hypophysitis secondary to PD-1/PD-L1 blockade: insights from four cases: Endocrinol Diabetes Metab Case Rep, 2019; 2019; 19-0102
17.. Zeng MF, Chen L, Ye HY, Primary hypothyroidism and isolated ACTH deficiency induced by nivolumab therapy: Case report and review: Medicine (Baltimore), 2017; 96; e8426
18.. Marchand L, Paulus V, Fabien N, Nivolumab-induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response: J Thorac Oncol, 2017; 12; e182-84
19.. Ohara N, Ohashi K, Fujisaki T, Isolated adrenocorticotropin deficiency due to nivolumab-induced hypophysitis in a patient with advanced lung adenocarcinoma: A case report and literature review: Intern Med, 2018; 57; 527-35
20.. Ariyasu H, Inaba H, Ota T, Thyrotoxicosis and adrenocortical hormone deficiency during immune-checkpoint inhibitor treatment for malignant melanoma: In Vivo, 2018; 32; 345-51
21.. Takebayashi K, Ujiie A, Kubo M, Isolated adrenocorticotropic hormone deficiency and severe hypercalcemia after destructive thyroiditis in a patient on nivolumab therapy with a malignant melanoma: J Clin Med Res, 2018; 10; 358-62
22.. Oristrell G, Baneras J, Ros J, Munoz E, Cardiac tamponade and adrenal insufficiency due to pembrolizumab: A case report: Eur Heart J Case Rep, 2018; 2; yty038
23.. Sato Y, Tanaka Y, Hino M, A case of nivolumab-induced isolated adrenocorticotropic hormone (ACTH) deficiency: Respir Med Case Rep, 2019; 26; 223-26
24.. Takeno A, Yamamoto M, Morita M, Late-onset isolated adrenocorticotropic hormone deficiency caused by nivolumab: A case report: BMC Endocr Disord, 2019; 19; 25
25.. Furubayashi N, Negishi T, Uozumi T, Isolated adrenocorticotropic hormone deficiency potentially induced by nivolumab following pseudo-progression in clear cell renal cell carcinoma: A case report: Mol Clin Oncol, 2019; 10; 304-8
26.. Ohara N, Kobayashi M, Ohashi K, Isolated adrenocorticotropic hormone deficiency and thyroiditis associated with nivolumab therapy in a patient with advanced lung adenocarcinoma: A case report and review of the literature: J Med Case Rep, 2019; 13; 88
27.. Pierrard J, Petit B, Lejeune S, Seront E, Isolated adrenocorticotropic hormone (ACTH) deficiency and Guillain-Barré syndrome occurring in a patient treated with nivolumab: BMJ Case Rep, 2019; 12; e230848
28.. Yamagata S, Kageyama K, Takayasu S, Progression of hypopituitarism and hypothyroidism after treatment with pembrolizumab in a patient with adrenal metastasis from non-small-cell lung cancer: Intern Med, 2019; 58; 3557-62
29.. Tanaka S, Kushimoto M, Nishizawa T, Isolated ACTH deficiency during single-agent pembrolizumab for squamous cell lung carcinoma: A case report: Clin Diabetes Endocrinol, 2020; 6; 1
30.. Bekki T, Takakura Y, Kochi M, A case of isolated adrenocorticotropic hormone deficiency caused by pembrolizumab: Case Rep Oncol, 2020; 13; 200-6
31.. Iglesias P, Sánchez JC, Díez JJ, Isolated ACTH deficiency induced by cancer immunotherapy: A systematic review: Pituitary, 2021 [Online ahead of print]
32.. Cano Megías M, Muñoz Delgado EG, Bone and metabolic complications of urinary diversions: Endocrinol Nutr, 2015; 62; 100-5
33.. Charmandari E, Nicolaides NC, Chrousos GP, Adrenal insufficiency: Lancet, 2014; 383; 2152-67
34.. Higham CE, Johannsson G, Shalet SM, Hypopituitarism: Lancet, 2016; 388; 2403-15
35.. Hollander-Rodriguez JC, Calvert JF, Hyperkalemia: Am Fam Physician, 2006; 73; 283-90
36.. Yamamoto T, Fukuyama J, Hasegawa K, Sugiura M, Isolated corticotropin deficiency in adults. Report of 10 cases and review of literature: Arch Intern Med, 1992; 152; 1705-12
37.. Seethapathy H, Rusibamayila N, Chute DF, Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors: Nephrol Dial Transplant, 2020 [Online ahead of print]
38.. Yano S, Ashida K, Sakamoto R, Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency: Eur J Cancer, 2020; 130; 198-203
39.. Lupu J, Pages C, Laly P, Transient pituitary ACTH-dependent Cushing syndrome caused by an immune checkpoint inhibitor combination: Melanoma Res, 2017; 27; 649-52
40.. Sekizaki T, Kameda H, Oba C, Nivolumab-induced hypophysitis causing secondary adrenal insufficiency after transient ACTH elevation: Endocr J, 2019; 66; 937-41
Figures
Tables
 Table 1.. Serial changes in levels of morning plasma adrenocorticotropic hormone and cortisol, serum electrolytes, and blood eosinophils, immediately before and during pembrolizumab therapy in 2019. All blood samples were collected in the morning.
Table 1.. Serial changes in levels of morning plasma adrenocorticotropic hormone and cortisol, serum electrolytes, and blood eosinophils, immediately before and during pembrolizumab therapy in 2019. All blood samples were collected in the morning. Table 2.. Endocrinological investigation.A: Rapid adrenocorticotropic hormone stimulation test.
Table 2.. Endocrinological investigation.A: Rapid adrenocorticotropic hormone stimulation test. . B: Corticotropin-releasing hormone (CRH)/growth hormone-releasing factor (GRF)/thyrotropin-releasing hormone (TRH)/luteinizing hormone-releasing hormone (LHRH) stimulation test (November 2019).
. B: Corticotropin-releasing hormone (CRH)/growth hormone-releasing factor (GRF)/thyrotropin-releasing hormone (TRH)/luteinizing hormone-releasing hormone (LHRH) stimulation test (November 2019). . C: Growth hormone-releasing peptide 2 (GHRP-2) stimulation test performed in November 2019.
. C: Growth hormone-releasing peptide 2 (GHRP-2) stimulation test performed in November 2019. Table 3.. Laboratory findings on admission in November 2019.
Table 3.. Laboratory findings on admission in November 2019. Table 4.. Summary of reports of patients who exhibited isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) associated with cancer treatment with programmed cell death 1 inhibitors.
Table 4.. Summary of reports of patients who exhibited isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) associated with cancer treatment with programmed cell death 1 inhibitors. Table 1.. Serial changes in levels of morning plasma adrenocorticotropic hormone and cortisol, serum electrolytes, and blood eosinophils, immediately before and during pembrolizumab therapy in 2019. All blood samples were collected in the morning.
Table 1.. Serial changes in levels of morning plasma adrenocorticotropic hormone and cortisol, serum electrolytes, and blood eosinophils, immediately before and during pembrolizumab therapy in 2019. All blood samples were collected in the morning. Table 2.. Endocrinological investigation.A: Rapid adrenocorticotropic hormone stimulation test.
Table 2.. Endocrinological investigation.A: Rapid adrenocorticotropic hormone stimulation test. . B: Corticotropin-releasing hormone (CRH)/growth hormone-releasing factor (GRF)/thyrotropin-releasing hormone (TRH)/luteinizing hormone-releasing hormone (LHRH) stimulation test (November 2019).
. B: Corticotropin-releasing hormone (CRH)/growth hormone-releasing factor (GRF)/thyrotropin-releasing hormone (TRH)/luteinizing hormone-releasing hormone (LHRH) stimulation test (November 2019). . C: Growth hormone-releasing peptide 2 (GHRP-2) stimulation test performed in November 2019.
. C: Growth hormone-releasing peptide 2 (GHRP-2) stimulation test performed in November 2019. Table 3.. Laboratory findings on admission in November 2019.
Table 3.. Laboratory findings on admission in November 2019. Table 4.. Summary of reports of patients who exhibited isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) associated with cancer treatment with programmed cell death 1 inhibitors.
Table 4.. Summary of reports of patients who exhibited isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) associated with cancer treatment with programmed cell death 1 inhibitors. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943420
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942824
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








